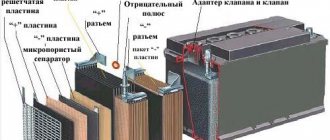
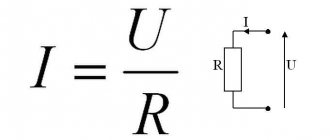Application examples
High temperature insulators.} In the form of porcelain beads for insulating the ends of heating coils. The scale-like design of the beads allows them to bend without exposing the conductor. Sometimes the heating coil is hidden with protective porcelain beads.
Housing of a mercury arc lamp from a light beam oscilloscope. The frame is made of aluminum alloy, the black body is carbolite, porcelain beads insulate the conductors that connect the lamp. The lamp gets very hot during operation. Nearby is a pile of cylindrical porcelain beads from various heaters.
Porcelain bead insulated conductors for operation near the high power xenon arc lamp of a film projector
Electrical parts. If you look inside the lamp socket, the part that contains the connection lamellas is most likely made of porcelain; it can work for a long time at the elevated temperature of an incandescent lamp without losing its properties. Fuse housings, sockets, lamp contact holders - wherever there is a danger of heating, porcelain has no competition.
The socket and socket slat holders are made of porcelain. The black body of the cartridges is carbolite.
Insulators on poles. The photo shows an insulator from a pole that was removed during the reconstruction of the line. Thirty years of sun, wind, bird droppings, rain, and frost did not affect the porcelain at all; it still looks like new; it was enough to wash the insulator with soap. (The service life of porcelain products is limited due to the appearance of microcracks during use.)
Porcelain power line insulators. Between the porcelain insulator and the steel hook there is a polyethylene sleeve to protect the porcelain from cracks. The disk shape of the insulators allows water to drain without forming a continuous layer that closes the conductor to the support. Porcelain insulators, unlike glass, are opaque, which makes it difficult to visually inspect the insulator for cracks.
Powerful resistors have a porcelain tube base. The green resistor has a winding hidden under the enamel.
Spark plugs from an internal combustion engine. The central electrode is insulated with porcelain. No other dielectric is capable of withstanding prolonged exposure to temperature, pressure, and fuel inside the combustion chamber.
Advantages and disadvantages of homemade liquid
The distillation process is considered almost the only way to effectively purify water, regardless of its origin. Unlike other filter systems, the composition of the water does not play a role here, and the result is not affected by the main distillation parameters: pressure, temperature.
This water has the following beneficial properties when consumed internally:
- helps fight excess weight;
- cleanses the kidneys and liver of salt deposits;
- restores kidney function;
- increases immunity;
- does not cause allergies;
- relieves intoxication of the body.
But regularly drinking distilled water can be harmful to your health. This is due to the fact that in large quantities it can disrupt water-salt metabolism, hormonal levels and lead to dental pathologies due to a lack of minerals.
In addition, distilled water does not taste particularly pleasant and causes a feeling of thirst.
Application examples
Housings for radio tubes, lighting lamps, fuses.
Glass and porcelain power line insulator that has worked outdoors for over 30 years.
Quartz tubes - housings of heaters, electric grills
A piece of technical quartz glass. A large number of bubbles are visible in the glass.
A typical sign (but not obligatory!) of technical quartz glass is a large number of bubbles in the direction of glass extrusion. More expensive optical quartz glass is completely transparent. The end of this glass is white, without a green tint.
Housings for low-power semiconductor diodes, insulators for radio element leads.
The housings of these semiconductor diodes are made of glass.
Disadvantages: Fragile, cannot withstand impacts. Some types of glass crack when exposed to sudden, uneven heating.
Interesting facts about glass
Here it is worth mentioning additionally about sapphire glass, tempered glass and chemically tempered glass. In advertising descriptions of many electronic devices for mass consumption, you can find references to these types of glass.
Sapphire glass is not formally glass (it is not amorphous like glass, but crystalline), but due to its external similarity it is called that. Sapphire glass is thin plates of leucosapphire (pure Al2O3} - aluminum oxide). Leucosapphire is harder than ordinary glass, therefore it is used to protect optics from abrasive abrasion by dust grains in military equipment and in expensive household devices. The glass of a sapphire wristwatch will remain scratch-free longer. At the same time, it is difficult to obtain large-sized sapphire crystals at a reasonable price, so we will not see tablets with sapphire crystal soon.
Strained glass. Glass resists compression well and poorly against tension. You can increase the mechanical strength of glass by tempering it - the glass is heated to high temperatures and cooled sharply and evenly. As a result, mechanical stresses are formed in the glass, which increase mechanical strength. Most often, glass is tempered for safety. Ordinary glass, if thrown with a stone, breaks into several fairly large fragments, which can cause serious injury. When broken, tempered glass produces many small fragments, which are much safer. Therefore, all (Except the windshield, otherwise it would be destroyed by the first pebble flying from under the wheels. The windshield is three-layered for safety - the middle layer is made of a polymer film with glue. Upon impact, all the fragments are glued to the film.) glass in a car, in shopping centers, is glass furniture shelves are hardened. A product made of tempered glass cannot be processed; if you try to trim a glass shelf for a bathroom, it will crumble into crumbs with a pop, so tempering is carried out after processing. A classic demonstration of the properties of tempered glass are Batavian tears.
Chemically tempered glass. For example, the often mentioned Gorilla glass. For thin glass plates, the thermal tempering method is not suitable, so the glass plates are processed in a solution that, for example, replaces the sodium ion with a potassium ion. Since the potassium ion is larger, the surface layers of the glass seem to be “bursted” with larger atoms in the lattice, creating just the required mechanical stresses. As a result, this glass is stronger and more resistant to scratches.
Heat-resistant glass. Regular window glass expands greatly when heated. If the heating is uneven, then parts of the glass will create mechanical stress due to different expansion, which can lead to cracking. By introducing additives, the coefficient of thermal expansion of glass is reduced, obtaining heat-resistant varieties. Such glasses do not form cracks when heated unevenly. Quartz glass is the coolest in this regard, which is why heater housings in electric grills are made from it.
Pure distilled water
Pure distilled water in the absence of air does not dissolve lead, since the positive potential of this metal is only slightly greater than that of hydrogen. Ordinary drinking water containing calcium and magnesium bicarbonates, as well as sulfate, forms a thin and hard layer of lead carbonate and sulfate (P) on the metal surface, preventing dissolution.
Pure distilled water conducts electricity to a small extent.
Pure distilled water is practically a dielectric. This can be shown using the following experiment: if you connect a bathtub with distilled water in which metal plates are immersed in series with an incandescent lamp, and turn on the lamp and the bathtub in the network, then the lamp does not light. It turns out that a solution of sugar in water also does not conduct current. If you add a few drops of acid into a bath of water using a pipette, the lamp lights up brightly. This means that a solution of acid in water is a good conductor of current.
Chemically pure distilled water has negligible conductivity. This can be easily verified experimentally by constructing an electrical circuit consisting of a current source, an ammeter and electrodes immersed in a glass vessel with distilled water. In this case, no electric current flows in the circuit.
Pure distilled water can be obtained by distillation, but depending on the still, it may contain traces of some elements.
Pure distilled water can be obtained by distillation, but depending on the still, it may contain traces of some elements.
The purest distilled water contains 20,000 - 30,000 dust particles in 1 ml.
A liter of pure distilled water at 22 contains 1 – 10 – 7 g of H – ions and 1 – 10 – 7 OH ions.
It is not advisable to subject pure distilled water to electrolysis.
For information on obtaining optically pure distilled water, see S yy rny K.
When alkalis are added to pure distilled water, a certain amount of OH ions will appear in it, which will lead to alkalization of the water.
We conclude that pure distilled water, organic solvents (alcohol and acetone), as well as solutions of salts in organic solvents do not conduct electric current.
The distillation apparatus is used to obtain pure distilled water by distilling it. The device is assembled on polished sections.
Acidification will become more noticeable if you add a slightly stronger acid than carbonic acid, such as hydrochloric acid, to clean distilled water. Carbonic acid disintegrates into ions only partially, and hydrochloric acid completely; therefore, even if you add an equal amount of these acids to the water (say, 1 mg each.
| Temperature dependence of the frequency-independent coefficient a/f2 of ultrasound attenuation in water. |
Mica
Mica . Natural layered material, has heat resistance, strength, and is an excellent dielectric. Micas are a large class of layered minerals, of which muscovite and sometimes biotite and phlogopite are used in technology.
In English, mica is Mica, hence the derivative names of materials based on mica - micanites, micalenta, micafolium, micalex, etc.
Mica extracted from the mine is disassembled and sorted. Large pieces are manually split into plates - this is how plucked mica - transparent, homogeneous plates. This mica is of the highest quality and is used for critical applications - in vacuum technology, radiation input/output windows, etc. Unfortunately, large, uniform pieces of mica without defects are rare, so mica plates of different shapes are glued together to form micanite . If you use fabric (fiberglass, paper) as a substrate for gluing mica plates, you get mica tape, micafolium, steklomicanite . Very fine mica waste is ground up and cast into a mesh in the form of an aqueous pulp, just like paper. After removing the water, the mica particles stick together into a single sheet - mica paper (mica, mica-plastic) . The resulting fabric can be impregnated with an organic binder for strength. The flexibility of mica paper allows it to be wound as insulation. You can also get rods and tubes by winding. If mica is impregnated with molten glass, the resulting durable material is called micalex.
Mica, ground into dust, is a component of pigments and, due to its “scale”, gives a pearlescent effect. Biotite is mainly used in pigments.
Synthetic material - fluorphlogopite (synthetic mica) is mica (phlogopite) where -OH groups are replaced by fluorine. Fluorphlogopite is more durable and thermally resistant, it also looks like mica, also layered but absolutely transparent/white, and not yellowish like natural mica. Alas, I haven’t encountered this material in person yet.
Application examples
Structural elements for holding heating elements in hair dryers, heaters, fan heaters, soldering irons, etc.
Heaters for household fan heaters. The design on the left is less material-intensive, but much less reliable, especially under mechanical loads.
As a protective window for the output of microwave radiation from the magnetron in microwave ovens. (usually when food gets on mica, it becomes charred and becomes a conductor and begins to spark violently, causing microwave owners to throw away the microwave in fear, although it is enough to cut out a plate from a mica sheet and replace the window.)
Mica window in the microwave. Sometimes there are plastic ones, but only on models without a grill.
Due to the fact that thin mica plates do not allow gases to pass through, but energetic charged particles do, mica windows are used in the designs of alpha and beta particle counters.
Used in radio tube designs - it holds the electrodes in place.
The octagonal plate is made of mica.
Used as a material for mica capacitors. Mica acts as a dielectric, and electrodes are conductive metal deposits on mica plates. This type of capacitor is becoming less and less common, replaced by capacitors based on polymer films. Mica capacitors can operate at high temperatures.
Mica capacitors made in the USSR half a century ago.
Mica plates in a capacitor. Metallization on the plates forms the linings.
Before the advent and widespread use of thermally conductive insulating gaskets made of polymer materials, such as Nomacon, mica plates were used for electrical insulation of components while maintaining thermal contact, for example, when it was necessary to attach several transistors, the housings of which were under different voltages, to one radiator.
Plates of natural plucked mica.
Natural mica is transparent. Mica materials obtained by processing natural mica are usually opaque.
Water and electric current
In order for a substance to conduct electric current, it must contain charged particles that can move freely through its entire volume under the influence of an applied electric field. In metal conductors, for example, such charged particles are free electrons, and in electrolytes - positively and negatively charged ions.
Dielectrics do not conduct direct electric current at all, since although there are charged particles in their structure, they are connected to each other and cannot move freely, forming a current.
But even dielectrics allow alternating current to pass through, this is called displacement current, for example, a capacitor in an alternating current circuit at a certain frequency will conduct current as if it were a conductor.
Regular unpurified water
As for ordinary water (river, tap, especially sea water, etc.), it always contains dissolved minerals, which, under the influence of an applied electric field, disintegrate into ions that can move as in an electrolyte.
For this reason, ordinary untreated water conducts current, behaving like a weak electrolyte. If you try to pass a current through such water, then for a short time it will flow through it, although weakly.
Theoretically perfectly pure water
Theoretically, if water is completely cleared of impurities, that is, absolutely all substances are removed from its volume, including salts, gases, and acid residues, then it will become a dielectric and behave as an insulator.
There will be no ions in it that can move under the influence of an electric field and form a current, and the water molecules themselves are electrically neutral. Such water could be used, for example, as a dielectric between the plates of a capacitor.
Real distilled water
But in reality, even distilled water (water purified by evaporation followed by condensation of steam) is not absolutely pure.
There is a Russian GOST 6709-72, which determines the mass concentration of residual impurities in such distilled water - no more than 5 mg per liter, and the minimum resistivity is not less than 2 kOhm * m.
That is, a cube of distilled water with a side 1 meter long, with electrodes attached to it along the edges, will have a resistance of at least 2 kOhm. And if you imagine distilled water spilled on the floor, say, in the volume of one glass (200 ml), then its resistance at best will be 200 kOhm. We can say that it is practically a dielectric.
There is no point in trying to use such water as a DC conductor. From this point of view, distilled water does not conduct electricity. It is usually used to correct the density of electrolytes.
Why you should be wary of any water coming into contact with electricity
However, it is not for nothing that people are afraid of contact of any water with electricity, especially with alternating voltage from an outlet. Even mains voltage from a wire dropped into a puddle of water that a person may accidentally step on can cause milliampere alternating current, which will be enough to cause harm to the body.
The human body and the phase from the socket, connected through a puddle of spilled water, form a circuit with reactive elements, and if a person in such a situation accidentally touches a grounded object, he will receive an electric shock. This is why it is necessary to avoid contact of electricity with water. As you understand, with distilled water the risk of harm is less, but it still remains. Therefore, it is better to avoid contact of any water with electrical appliances.
Source
Interesting facts about mica
Previously, several centuries ago, when they did not know how to make thin window glass, translucent structures were made by splitting natural mica. Since large pieces of mica without defects were rare, the windows also took on a bizarre shape.
Mica instead of glass in a window frame. From the exhibition of the Krasnoyarsk Museum of Local Lore.
Mica is a fairly soft material; a mica plate (like most materials based on it) can be easily cut with scissors. Due to its layered nature, gluing mica is an unreliable task, the adhesion force between layers is low, therefore, during production, mica parts are fastened mechanically - rivets, eyelets, screws, etc.
Electrical connections to the heating element are made with hollow rivets.
Application examples
Chip housings, usually for critical applications.
Processor cases used to be made of ceramic, but increased heat generation and price competition forced them to abandon this material. It was with the ceramic housing of the processors that the joke about the new Russian and the tiles in the bathroom from Intel was connected.
Housings of electrovacuum devices.}
The housing of the magnetron vacuum flask is made of copper and aluminum oxide ceramics. The ceramics are visible in the photo, there is a purple band between the cap and the body.
Aluminum oxide ceramics are very hard and, like many ceramics, are processed with diamond tools. A piece of a ceramic chip casing is an excellent tool for writing messages on a car windshield; it leaves clear, even scratches no worse than a glass cutter.
This type of ceramic is dense, does not absorb moisture, holds a vacuum, and does not crack due to sudden temperature changes and thermal shock. At the same time, the adhesion of metal films to the surface is high, making it possible to make tracks on ceramics and hermetically weld metal parts.
Outwardly, beryllium ceramics are very similar - it is superior to aluminum oxide ceramics in terms of maximum operating temperature and thermal conductivity (comparable to metals!), but due to the high cost and toxicity of dust from it, it is rarely used.
Asbestos
A unique, unsurpassed class of materials. Natural fiber, “mountain flax”. It is a fire-resistant dielectric. It has been used in a variety of applications, ranging from reinforcing additives in polymers to insulation of heating devices. Available in the form of sheets (asbestos cardboard), threads, yarn. Most often it is used precisely as a heat insulator, as a dielectric only in low-voltage (up to 1 kV) installations.
Widely used in construction. Slate is cement reinforced with asbestos fibers, an almost eternal material. Its low cost and fire resistance were highly valued. But there is one thing:
Asbestos is a carcinogen. Moreover, it is a class 1 carcinogen (from IARC), on a par with arsenic and formaldehyde. (The degree of danger of various types of asbestos is a debatable issue, and there is no unanimous opinion on this matter.) Long-term observation has shown that asbestos products generate dust from fibers, which, when inhaled, can provoke a lung disease - asbestosis. First of all, workers at asbestos mining and processing enterprises are at risk. Those who use asbestos products on a daily basis are less at risk. In other cases, there is no reason to panic; if your dacha roof is covered with slate, and the stove in the bathhouse is covered with asbestos cardboard, then you will most likely die not from asbestos, but from diseases of the cardiovascular system (mortality statistics).
{A piece of asbestos cardboard and an old dirty asbestos cord. Asbestos is very soft to the touch and does not ripple like fiberglass.
Asbestos and asbestos products are still widely produced, since in some applications there is simply nothing (or too expensive) to replace asbestos without losing its properties. Asbestos is an excellent material for constructing experimental devices containing heaters or hot parts. On a piece of asbestos cardboard, you can safely heat parts up to 1000°C with a gas burner, while it will retain its shape. Asbestos thread is convenient for tightening nichrome in heaters.
Magnetic amplifier and current shunt from the 50-VUK-120-1 power supply on a board made of asbestos-based material.
Fable (from Wikipedia): There has long been a legend about how Akinfiy Demidov brought Peter I a beautiful snow-white tablecloth from his Ural factory. During the meal, he defiantly knocked over a bowl of soup on the tablecloth, poured out a glass of red wine, and then crumpled the tablecloth and threw it into the fireplace. Then, taking it out of the fire, he showed it to the king: not a single spot remained on it. This tablecloth was made from Ural chrysotile asbestos. Indeed, Demidov serf workers achieved perfection in the manufacture of asbestos fabrics. They were used to make openwork ladies' hats, gloves, wallets, handbags and lace. They did not require washing, they were thrown into the fire, and after a few minutes of cooling they could be worn again.
Water as a substance
The water molecule, as we know, consists of one oxygen atom and two hydrogen atoms. Its formula is written as follows: H 2 O. This substance can have three states: solid - in the form of ice, gaseous - in the form of steam, and liquid - as a substance without color, taste and smell. By the way, this is the only substance on the planet that can exist in all three states simultaneously under natural conditions. For example: at the Earth's poles there is ice, in the oceans there is water, and evaporation under the sun's rays is steam. In this sense, water is anomalous.
Water is also the most abundant substance on our planet. It covers the surface of planet Earth by almost seventy percent - these are oceans, numerous rivers with lakes, and glaciers. Most of the water on the planet is salty. It is unsuitable for drinking and for farming. Fresh water makes up only two and a half percent of the total amount of water on the planet.
Water is a very strong and high-quality solvent. Thanks to this, chemical reactions in water occur at tremendous speed. This same property affects the metabolism in the human body. It is a well-known fact that the adult body is seventy percent water. In a child this percentage is even higher. By old age, this figure drops from seventy to sixty percent. By the way, this feature of water clearly demonstrates that it is the basis of human life. The more water in the body, the healthier, more active and younger it is. That’s why scientists and doctors from all countries tirelessly insist that you need to drink a lot. It is water in its pure form, and not substitutes in the form of tea, coffee or other drinks.
Water shapes the climate on the planet, and this is not an exaggeration. Warm ocean currents warm entire continents. This happens due to the fact that water absorbs a lot of solar heat, and then releases it when it begins to cool. This is how it regulates the temperature on the planet. Many scientists say that the Earth would have cooled down and turned into stone long ago if it were not for the presence of so much water on the green planet.
Water
This is completely counterintuitive, but this point is included here to blow your mind. Water does not conduct current! Everywhere they teach that water is a good conductor of electricity, and this is usually true. But very pure deionized water, which contains nothing but H2O, does not conduct current - its resistivity is 18 MOhm*cm. The water that conducts current is not pure enough. Measuring electrical conductivity is a fairly simple way to assess the quality and purity of water. (Relevant for direct current and low frequency alternating current.)
Having highly polar and mobile molecules, water is not only an insulator, but also has a very high dielectric constant - about 81 at room temperature (for most conventional dielectrics it does not exceed 20-30). Capacitive humidity meters are based on this: a small amount of water between the plates of the capacitor dramatically increases its capacity.
Unfortunately, water is an excellent solvent, and substances dissolved in it usually form electrolytes. Once distilled water is left in the air, it dissolves carbon dioxide, forming an electrolyte - a weak solution of carbonic acid. Water can also dissolve the walls of the vessel in which it is located. The slightest admixture of salts, especially chlorides and sulfides of sodium, potassium, and calcium, sharply increases the conductivity of water. Therefore, in practice, water is no good as a dielectric.
A bottle of deionized water from a radio store. Printed circuit boards of electronic devices should only be washed with distilled or deionized water, otherwise the salts contained in the water can cause trouble.
Project “What substances conduct electricity when dissolved in water”
Electric flow is the result of the movement of electrically charged particles (electricity) under the influence of the forces of an electric field applied to them. Pure water is a poor conductor of electricity, but certain elements dissolved in it allow it to conduct electricity. When dissolved, such substances form ions (charged particles) that transfer charge within the solution. Solutions with this property are called electrolytes. The more ions in a solution, the higher its conductivity. Nonelectrolytes are solutions that do not contain ions and do not conduct current. Electrolytes can be weak or strong. It depends on how they are ionized: completely or partially.
The conductivity of a solution can be measured using a conductivity device consisting of two metal electrodes, usually spaced 1 cm apart (which is why it is measured in microsiemens or millisiemens per centimeter). A constant voltage is applied to both electrodes. This causes an electric current in the solution. Because it is proportional to the number of ions in the water, conductivity can be measured. The higher the ion concentration, the higher the conductivity of the sample.
A conductivity device is commonly used in hydroponics, swimming pools, and water purification systems to monitor the amount of nutrients, salts, or contaminants.
A solution of some substances in water conducts electricity. These substances form ions when dissolved, and these ions carry charge through the solution. This project aims to build a device to identify which substances in solution can conduct electricity and which cannot.
The focus of this project is to create a device that would allow us to determine which substances, when dissolved, can conduct electricity - and what type of electrolyte they are in this case.
What we need:
- conductivity device;
- plastic cups;
- large paper clips;
- insulating tape;
- different types of water: distilled, mineral, carbonated;
- vinegar;
- sugar;
- salt.
Progress of the experiment:
- Experimenting with electricity at home requires care. Do not ingest the substances used in this experiment!
- Prepare different types of water.
- Prepare salt and sugar solutions by dissolving them in distilled water.
- Pour the liquid into a glass.
- Unfold the paper clips, securing them with electrical tape on opposite sides of the cup.
- Do not place the contacts directly into the solution, otherwise they will rust over time. Instead, place them on paper clips and dip the paper clips into the solution.
- Display the observation results in a table and graph. Depending on which conduction device you are using, note whether the LED lights are on and how bright they are. Rinse the cup and paper clips with distilled water between tests.
- If there is a spring nearby, test the water for conductivity. If it conducts electricity, think about what substances might be dissolved in it and where they might come from.
- Check the box corresponding to the light produced by the LED lamp. Depending on the brightness of the lamp, categorize liquids into strong, medium, weak electrolytes or non-electrolytes.
| Light intensity/liquid | Bright | Medium brightness | Weak | No light | Electrolyte type |
| Distilled | |||||
| From the tap | |||||
| Mineral | |||||
| Rainy | |||||
| Salt solution | |||||
| Sugar solution | |||||
| Carbonated | |||||
| Vinegar |
Conclusion:
What is electricity? What is an electrolyte? What is conductivity? What substances turned out to be good electrolytes based on the results of the experiment? Look at the label of your mineral water bottle. What substances in its composition do you think help conduct current? Look at the label on your bottle of sparkling water. What substances in its composition do you think help conduct electricity? The liquid paste inside flashlight batteries is electrolyte. Which of the tested substances could be used as such an electrolyte? Think about what other experiments with electricity at home you can do based on the project you completed.
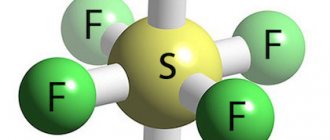
SF6 gas
Dielectrics can be gaseous. Dry air is a good dielectric, but in some applications its electrical insulating properties are insufficient. An example of a gaseous dielectric is sulfur hexafluoride or “sulfur hexafluoride”; it is heavier than air and has a breakdown voltage several times higher than that of air, which makes it possible to make an electric machine more compact. In addition, SF6 gas has arc-extinguishing properties and upon contact with an arc practically does not degrade, recombining back.
It’s quite a funny experience when, having inhaled helium, a person’s voice becomes higher; with SF6 gas it looks different - the voice becomes lower. Another video: Helium - sulfur hexafluoride pair Since SF6 gas is heavier than air, a light boat can float in it.