The service life of a gel battery, according to manufacturers, is at least 10 years. In practice, the need to repair or replace it becomes obvious after 3-4 years, when a decrease in capacity is observed or the electrolyte solution dries out. The product belongs to the category of maintenance-free elements, but experienced vehicle owners know how to restore a gel battery at home.
Diagram: what parts does a gel battery consist of?
Fundamental differences between gel batteries
Such batteries differ from lead-acid analogues in a number of technical characteristics and electrolyte composition. The binding solution in it is distilled water with the addition of silica gel, which tightly seals the contents of the case.
This allows you to avoid the fumes of chemicals, which affects the level of environmental friendliness of the device - it can be installed not only inside the car, but also in residential areas. In addition, due to the absence of gas emissions, their re-involvement in the work process is ensured and the service life of the product is increased.
Another advantage of the battery is the ability to place it in any position - on its side or upside down, while acid batteries need strictly vertical fixation.
Advantages of the gel battery design.
Batteries with gel electrolyte do not require deep recharging, charge quickly and maintain a service life in standby mode for 2 years.
Due to the increase in the number of charge-discharge cycles, more intensive use of such products is possible.
Among the negative features of such batteries are:
- Instability to low temperatures. At -50⁰C the electrolyte freezes and becomes brittle.
- Rapid failure when applying voltage above 15 V.
- High price.
Features of gel batteries
If we talk about the fundamental differences in comparison with classic liquid lead-acid batteries, they lie in the electrolyte itself, as well as some characteristics.
Gel batteries use sulfuric acid, silica gel (thickener) and distilled water. In fact, it is the same acid, only in a gel state.
In this case, the housing becomes sealed. There are no plugs for access to the banks to add electrolyte or distillate. The tightness made it possible to use such batteries in enclosed spaces. When charging, gas is not released, unlike when being serviced. There is also no consumption of the water component. There is no mention of any addition of fluids in the case of batteries using GEL technology.
There is an analogue of GEL batteries called AGM. They are not the same thing, contrary to popular belief. AGM uses fiberglass mats that are impregnated with liquid electrolyte.
The sealing factor has allowed gel batteries to gain a number of advantages, including:
- increased service life;
- no need to top up liquids;
- no gas emissions;
- safety for humans;
- ability to install in any position;
- do not require deep recharging;
- charge faster;
- in standby mode they do not lose service life for about 2 years;
- increased number of charge-discharge cycles.
There are only 3 significant drawbacks. This is high cost, instability to high voltage, and also a tendency to freeze at temperatures below -45-50 degrees Celsius.
When servicing, it is only important not to apply voltage exceeding 15 V. Well, winters with a temperature of -50 degrees are rare even for Russia.
Regarding the price, the issue is controversial. Buying a gel battery is fully justified. This is a battery with a solid lifespan and minimal intervention required.
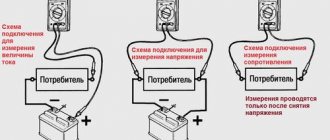
How to check a gel battery
If there are no obvious flaws, the device is checked using a voltmeter and a load fork, simulating the operation of the starter for 3-4 seconds. The load is connected after setting the voltage values at the terminals, and then an improvised motor is connected for a short period.
The rate of recovery of the electromotive force is assessed by observing the voltmeter needle, which should rise back.
A rapid drop in current indicates that the battery has almost used up its resource; a new device takes a long time to charge. If no changes occur, the voltage can be increased by gradually adding 5 V. During the operation, you should pay attention to ensure that the temperature of the device does not exceed 40-45⁰C.
How to measure the internal resistance of a battery
If we close the plus and minus of the battery, we get a short circuit current
Ie = U/Re, as if there is resistance
Re
. Internal resistance depends on the electrochemical processes inside the element, including current.
If the current is too high, the battery will deteriorate and may even explode. Therefore, do not short the plus and minus. Enough thought experiment.
The value of Re
can be estimated indirectly by changes in current and voltage across the load
Ra
. With a slight decrease in load resistance Ra to Ra‑dR, the current increases from Ia to Ia+dI. The voltage at the output of the element Ua=Ra×Ia decreases by the amount dU = Re × dI. Internal resistance is determined by the formula Re = dU / dI
To estimate the internal resistance of a battery or battery, I added a 12 ohm resistor and a toggle switch (the button is shown below in the diagram) to change the current by dI = 1.2 V / 12 Ohm = 0.1 A. At the same time, you need to measure the voltage on the battery or resistor R
.
You can make a simple circuit just to measure the internal resistance, similar to the one shown in the figure below. But it’s still better to first discharge the battery a little and then measure the internal resistance. In the middle, the discharge characteristic is flatter and the measurement will be more accurate. The result is an “average” value of internal resistance, which remains stable for quite a long time.
(Scheme description)
Example of determining internal resistance
We connect the battery and voltmeter. Voltmeter shows 1.227V
.
We press the button: the voltmeter shows 1.200V
.
dU = 1.227V - 1.200V = 0.027V Re = dU / dI = 0.027V / 0.1A = 0.27 Ohm
This is the internal resistance of the element at a discharge current of 0.5A
The tester does not show dU, but simply U. In order not to make mistakes in the mental calculation, I do this. (1) I press the button. The battery begins to discharge and the voltage U begins to decrease. (2) At the moment when the voltage U reaches a round value, for example 1.200V, I press the button and immediately see the value U+dU, for example 1.227V (3) New numbers 0.027V - and there is the desired difference dU.
As batteries age, their internal resistance increases. At some point, you will find that the capacity of even a freshly charged battery cannot be measured, since when you press the Start
The relay does not turn on and the clock does not start. This happens because the battery voltage immediately drops to 1.2V or less. For example, with an internal resistance of 0.6 ohms and a current of 0.5 A, the voltage drop will be 0.6 × 0.5 = 0.3 volts. Such a battery cannot operate at a discharge current of 0.5A, which is required, for example, for a ring LED lamp. This battery can be used at a lower current to power a watch or wireless mouse. It is by the large amount of internal resistance that modern chargers, like the MH-C9000, determine that the battery is faulty.
Internal resistance of a car battery
To evaluate the internal resistance of the battery, you can use a lamp from a headlight. It should be an incandescent lamp, for example, a halogen, but not an LED. A 60W lamp consumes 5A current.
At a current of 100A, the internal resistance of the battery should not lose more than 1 Volt. Accordingly, at a current of 5A, more than 0.05 Volts (1V * 5A / 100A) should not be lost. That is, the internal resistance should not exceed 0.05V / 5A = 0.01 Ohm.
Connect a voltmeter and a lamp in parallel to the battery. Remember the voltage value. Turn off the lamp. Notice how much the voltage has increased. If, say, the voltage increases by 0.2 Volt (Re = 0.04 Ohm), then the battery is damaged, and if by 0.02 Volt (Re = 0.004 Ohm), then it is working. At a current of 100A, the voltage loss will be only 0.02V * 100A / 5A = 0.4V
Using a light bulb you can also estimate the capacity of a car battery.
Is the gel recoverable?
The basis for battery restoration is the drying of moisture in the electrolyte. To check the liquid level in the cans, open the battery cover with a knife or other sharp instrument. When returning the cover to its original position, you must ensure that there is no dirt on the valves.
Because of this, they may not close, and the gel will dry out faster. In addition, the integrity of the device case and its contents is taken into account. The amount of charge stored in the battery must be at least 30% of the required value.
Basic rules for safe charging
The gel battery can be charged at home. The main thing is to ensure the supply of direct current. The procedure is carried out using a 12V charger, which is used only for gel devices.
You can make such chargers yourself. What distinguishes them from other products is the ability to control the incoming voltage. It is recommended to set this indicator at 0.1 of the battery power, the charging time will be about 12 hours. A device designed for acid-type devices cannot be used.
The battery is fully charged and is not used at a low energy level.
A long stay in a discharged state leads to a decrease in capacity. Regularly recharging the battery will prevent this situation.
How to check a watch battery with a multimeter?
Watch batteries may vary. For example, Chinese alarm clocks use a finger alarm clock, and sometimes a finger alarm clock. The watch is equipped with a button cell battery.
You can check this battery using a multimeter, placing one end of the probe against the plus and the other against the minus. The positive terminal is wide and covers a significant portion of the battery.
Thus, the voltage of this battery is 1.56 v.
How to restore a battery
The process of restoring a gel battery.
Gel batteries are more demanding to maintain, so refurbishing them requires great care. The following tools are used during the procedure:
- syringe;
- tweezers;
- screwdriver;
- Charger.
To restore electrolyte levels, you will need distilled water, which can be purchased at a pharmacy.
Filling distillate
The battery is disassembled by removing the top cover, after which the rubber valve caps are removed with tweezers. Then use a syringe to add 2 ml of distillate to each jar. The liquid must be added gradually as the water is absorbed into the gel. The restored level should completely cover the lead plates, and excess moisture is pumped out with the same syringe.
In some cases, instead of distilled water, electrolyte is poured into the battery. This will work if the battery is old and needs to be revived temporarily.
Recovery using long-term battery charging
After adding distilled liquid, the valves are returned to their place and closed with a lid, which is fixed with tape or glue, and a load is placed on top. This is necessary in order to prevent the valves from breaking due to the released gas.
When connecting the battery to the charger, set the voltmeter to 14-15 V and wait until the battery restores current consumption (within 14 hours). If this does not happen, the voltage is increased to 20 V.
It is important not to leave the battery unattended, since restoration of the electrolyte leads to a sharp drop in load and can cause smoke and burnout of the device. Therefore, as soon as current flows, the level of electromotive force is reduced to 14 V and the battery continues to be charged in the standard mode, i.e., with a charge equal to 0.1 of its nominal capacity.
Cyclic charging
The method is used for severe loss of capacity. It involves 3-4 repeating cycles of charging and discharging.
The first two are carried out under a high voltage of 25-30 V, which gradually decreases to 14 V as current consumption increases. For discharge, a 5-10 W light bulb is used, and care must be taken that the potential difference does not fall below 10, 5-11 V.
After recovery, the battery is charged in standard mode. If it is not possible to return full power to the battery, it is used with an incomplete charge or a new model is purchased.
How to check AA batteries for suitability
To check whether a battery is good, you need to measure the current it delivers. Depending on the chemical composition of the battery, it should be several amperes (see the symbols on the battery itself) and not decrease for several seconds. If the current value is small or drops noticeably, the battery can be disposed of.
Before checking the AA battery for functionality, prepare:
- multimeter or tester;
- 1.5 V light bulb;
- wiring;
- charger compatible with the batteries being tested (for nickel-cadmium batteries - 1.2 V).
First, the batteries to be tested must be completely discharged by connecting the light bulb observing the polarity (plus to plus, minus to minus). To do this, you can use wires or take a battery case of the appropriate format. When the light bulb dims, you should disconnect the battery, place it in the charger and charge it for the time specified in the instructions.
Next, you need to measure the voltage of the charged battery using a multimeter or tester, strictly observing the polarity (plus to plus, minus to minus). The measured voltage should be in the range of 1.1–1.4 V. For a full test, you need to hold the device terminals for 1 minute and see how much the voltage drops. The permissible voltage drop is 0.01–0.05 V per minute. If the voltage drops faster, the battery is bad. If the measured parameters meet the requirements, then you need to measure the current.
How to extend the device's operating life
Application of GEL (Gelled Electrolite) and AGM (Absorptive Glass Mat) technologies for the need to maintain a gel battery.
The service life of a gel battery depends on the operating conditions and care of it. It is not recommended to allow the battery to completely discharge.
If this happens, use a limited charge replenishment scheme - not 10%, but 5% of the declared capacity - and double the process time. The loss of energy intensity during such actions is less than with the standard method.
To avoid such situations, you should purchase a multimeter. For preventive purposes, they regularly check the correct fastening of the terminals and perform a full battery charging cycle once a quarter.
In what cases is battery restoration inappropriate?
Batteries are disposed of:
- When the cans swell, which is caused by the detachment of the gel from the plates. Such a defect is determined visually.
- When the contents of the case are destroyed as a result of prolonged exposure to high temperature. If during inspection of the battery there are extraneous sounds coming from it, the element cannot be “revived”. You can also enlighten the holes of the cans and make sure that the lead plates have lost their original appearance.
The right decision in this case would be to buy a new gel battery.