Conductors
Conductors are characterized by good electrical conductivity, that is, a large number of free electrically charged particles (electrons or ions) that can move under the influence of field forces along the conductor.
Conductors of the first kind
There are two kinds of conductors. Conductors of the first kind, in which only electrons can move, are metals. In metallic conductors, electrons located in the outer orbits of atoms are relatively weakly coupled with their nuclei, which is why some of the electrons separated from their nuclei move between the atoms, moving from the sphere of action of one nucleus to the sphere of action of another and filling the space between them like a gas. These electrons are usually called free electrons or conduction electrons. Free electrons are in a state of random (thermal) motion, in contrast to the positively charged metal ions that make up the core of the conductor, which have very low mobility and make only small oscillations around their average position.
Conductors of the second kind
In conductors of the second type, called electrolytes (aqueous solutions of acids, salts, alkalis and bases), under the influence of a solvent, the molecules of the substance break up into negative and positive ions, which, like electrons in metal conductors, can move throughout the entire volume of the conductor.
Conductors and dielectrics. Types of conductors
The electron has the least negative charge.
For reference: the electron charge is e0 = -1.6021766208*10-19 Coulomb
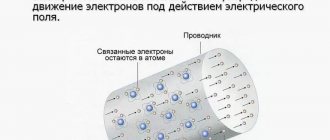
An electron (if it is weakly bound to the nucleus of an atom) can leave the atom, move into the interatomic space, enter the boundaries of another atom, etc. This phenomenon is most characteristic of metals. Metals always contain a huge number of electrons moving randomly in the interatomic space, called free (Figure 1).
Figure 1. Chaotic movement of electrons in a metal.
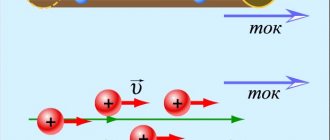
If we somehow order the movement of free electrons, that is, force them to move in one specific direction, then we will obtain an electric current in the metal (Figure 2).
Figure 2. The occurrence of current in a conductor.
Definition: Bodies with free electrons are called conductors of the first kind.
In conductors of the first type, the passage of electric current does not cause chemical changes in their substance. Conductors of the first kind include metals and their alloys. Conductors of the first type have found the widest application in electrical engineering and radio engineering. Wires, tires, capacitor plates, filaments of incandescent lamps and other conductive parts - all this is made from conductors of the first kind.
Definition: Conductors of the second type include solutions of acids, alkalis and salts.
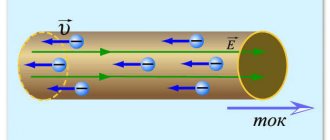
Conductors of the second type are often called electrolytes. In the electrolyte, a continuous process of formation of negatively and positively charged molecules (ions) occurs. Electric current in an electrolyte represents the ordered movement of these ions (and not electrons, as was the case in conductors of the first kind).
Figure 3. Current in conductors of the second type (electrolytes).
Finally, there is a large group of substances that have neither free electrons nor ions. In such substances, under normal conditions, electric current cannot pass, and they are called dielectrics (porcelain, rubber, mica, glass, etc.).
Definition: Dielectrics are substances that do not have free electrons.
Dielectrics are widely used in modern electrical engineering as insulators (porcelain insulators on power lines, rubber coatings on wires, mica spacers, etc.).
DID YOU LIKE THE ARTICLE? SHARE WITH YOUR FRIENDS ON SOCIAL NETWORKS!
Related materials:
- The structure of matter or what matter is made of
- Electronic structure of the atom
- Electric current in a conductor
- Resistance to electric current.
Comments
Zalim 05/02/2019 05:15 Helpful
Quote
Update list of comments
Add a comment
Dielectrics
Substances in which the number of free electrons is negligible are called non-conductors (dielectrics or insulators). These include gases, some liquids (mineral oils, varnishes) and almost all solids, with the exception of metals and coal.
The best non-conductor of electric current is vacuum. Gases, including air, are also good insulators.
SUBJECT OF ELECTROCHEMISTRY.
Arrhenius' theory of electrolytic dissociation. Ostwald's law of dilution. Causes of dissociation.
Subject of electrochemistry. Conductors of the first and second kind. Faraday's laws.
Electrochemistry is a branch of physical chemistry that studies the laws of the relationship between chemical and electrical phenomena. The main subject of electrochemistry is the processes occurring on electrodes when current passes through solutions - electrode processes . Two basic sections of electrochemistry can be distinguished: the thermodynamics of electrode processes, covering the equilibrium states of electrode-solution systems, and the kinetics of electrode processes, which studies the laws of the occurrence of these processes over time. Electrochemistry also studies the theory of electrolytes.
Electrochemistry is very important because the laws of electrochemistry are the theoretical basis for the development of important technical processes - electrolysis and electrosynthesis , ᴛ.ᴇ. obtaining chemical products on electrodes when current passes through solutions (production of chlorine and alkalis, production and purification of non-ferrous and rare metals, electrosynthesis of organic compounds). An important area of practical application of electrolysis is electroplating - electroplating with metals. Another important area of technology, which is based on electrochemical processes, is the creation of chemical current sources (galvanic cells, including batteries), in which a chemical reaction is used as a source of electric current.
Electrochemical methods of chemical analysis (electroanalysis, conductometry, potentiometry, polarography, etc.) have received great development.
The emergence of electrochemistry as a science is associated with the names of Galvani, Volta and Petrov, who at the turn of the 18th and 19th centuries. discovered and studied electrochemical (galvanic) elements. Devi and Faraday in the first half of the 19th century. studied electrolysis. The rapid development of electrochemistry at the end of the 19th century. associated with the emergence of the theory of electrolytic dissociation by Arrhenius (1887) and with the work of Nernst on the thermodynamics of electrode processes. Arrhenius's theory was developed by Debye and Hückel (1923), who developed the electrostatic theory.
Recent decades have been characterized by the rapid development of electrochemical kinetics, the study of the phenomena of overvoltage, corrosion, galvanic coatings, etc.
Solid and liquid conductors, the passage through which an electric current does not cause the transfer of matter in the form of ions, are called conductors of the first kind . Electric current in conductors of the first kind is carried out by a flow of electrons ( electronic conductivity ). Such conductors include solid and liquid metals and some non-metals (graphite, zinc and lead sulfides).
Substances through which an electric current passes causes the movement of the substance in the form of ions ( ionic conductivity ) and chemical transformations at the points of entry and exit of the current (electrochemical reactions) are called conductors of the second kind . Typical conductors of the second kind are solutions of salts, acids and bases in water and some other solvents, molten salts and some solid salts. As a rule, in conductors of the second type, electricity is carried by positive (cations) and negative (anions) ions, however, some solid salts are characterized by unipolar conductivity, that is, current carriers in them are ions of only one sign - cations (for example, in AgCl) or anions (BaCl2, ZrO2+CaO, solutions of alkali metals in liquid ammonia).
The division of conductors based on the type of conductivity (electronic or ionic) is conditional. Solid substances with mixed conductivity are known, for example Ag2S, ZnO, Cu2O, etc. Posted on ref.RF In some salts, when heated, a transition from ionic conductivity to mixed conductivity (CuCl) is observed.
Conductors of the second kind are called electrolytes . These can be pure substances or solutions. Substances whose solutions conduct electric current are often called electrolytes. These solutions are called electrolyte solutions.
Conductors and dielectrics in an electric field
However, under certain conditions, for example in a strong electric field, dielectric molecules split into ions, and a substance that was an insulator in the absence of an electric field or in a weak field becomes a conductor. The electric field strength at which ionization of dielectric molecules begins is called the breakdown strength (electric strength) of the dielectric. The amount of electric field strength that is allowed in a dielectric when used in an electrical installation is called the permissible strength. The permissible voltage is usually several times less than the breakdown voltage.
The electrical properties of gases are strongly influenced by pressure and temperature.
As an example, we give the values of breakdown voltage in sq. cm for some dielectrics: air - 30, mineral oil (transformer) - 50-150, electrical cardboard - 100, porcelain - 80-150, mica - 800-2000.
Conductors and dielectrics grade 8 video:
Conductors of the first and second kind
School textbook:
“In conductors of the first kind, electrons move between atoms and cations of the metal, which only oscillate around the equilibrium position. In conductors of the second type - electrolytes - the charge during the flow of electric current is carried by ions - particles of the substance itself. It is not the current (but, in the usual sense, the flow of electrons) that flows in the substance, but the substance itself moves, flows, pushed by the electric field. Moreover, particles of this substance - cations and anions - rush in different directions. Ions that have a positive charge move towards the negatively charged cathode (which is why they are called “cathode ions” - cations), and negative ions move towards the positively charged anode (anodic ions - anions).”
— — — — — — — —
Russian theory:
First, some general notes:
Metals do not have cations. Metal atoms do not “oscillate around an equilibrium position.” Charges don't exist. The “substance itself” is not pushed by the electric field. There is no electric field itself. Non-existent cations and anions do not “rush in different directions” under the influence of electrical forces. There is neither electrical attraction nor electrical repulsion, that is, there are no electrical forces.
Conductivity of metals. Metal atoms have contour grooves. When atoms stick together in the same grooves, they form continuous chains. Electrons slide along them. They shift down the slope of the electron pressure.
Electrons slide along the trough without energy loss, since they do not approach or move away from the trough. Difficulties arise when overcoming the joints of the gutters. Electrons can be pushed at the junctions by external pressure (electrical voltage) or rolling waves of radiation.
Conductivity of electrolytes. Pure mechanics also operates in an electrolyte. Features of the electrolyte are that:
* electrolyte molecules do not have contour grooves and therefore do not form conductive chains;
* an electrolyte molecule consists of an acidic component to which a metal atom or a hydrogen molecule is attached (molecular hydrogen is also a metal);
* the metal atom is almost always attached to the acidic component asymmetrically; therefore, when separated, its reactive force turns the acidic component in one direction, and under the influence of random shocks of neighboring molecules it can turn in the opposite direction; thus, the electrolyte molecule performs rotational movements;
* electrolyte – liquid (not solid or gaseous); Only in this state do the electrolyte molecules maintain contact with each other and can perform rotational movements.
The movement of electrons begins at the cathode.
Under the pressure of electron pressure, they move in groups to those electrolyte molecules that are adjacent to the cathode with their metal atoms (more precisely, those particles in the composition of the electrolyte molecules that have a convex groove). Acting like a wedge, the electrons tear these atoms away. The reactive force turns the remaining part of the molecule so that its opened trough turns in the opposite direction from the cathode. Electrons from the opened trough jump to the next molecule and again, like a wedge, tear off a metal atom from it. Under the action of an abstraction impulse, it approaches the acidic residue of the previous molecule and combines with it. Purely by chance, in the turmoil of turning particles, some of the reduced molecules turn out to be turned back towards the cathode. The process is repeated.
Thus, electrons move (jump) from molecule to molecule of the electrolyte and are directed from the cathode towards the anode. In this case, metal atoms make transitions from one acidic component to another in the opposite direction.
In this process there is no electron transfer between the electrolyte particles; acidic components remain in place; they just turn back and forth.
Electric current in conductors of the second kind
The electrolytic circuit in which current is generated always consists of a conductor of the first kind and a conductor of the second kind - the electrolyte.
The fact is that in conductors of the second kind, unlike conductors of the first kind, there are two force fields. One field is formed under the influence of electrostatic forces E, the other - under the influence of external forces Estor (ponderomator). Energy balance (the basic law of conservation and transformation of energy) can only be satisfied if these forces are equal!
Molecular kinetic theory is the basis for calculating the energy required to tear out an electron or transfer the energy of one atom to another.
The reactions that take place in various types of electrolytic cells give approximately the same number of joules for each individual act of chemical interaction between particles (i.e., the same number of joules per elementary charge). In most cases, about 1·10-19 or 2·10-19 J per elementary charge. Therefore, from a practical point of view, it is convenient to take the same value as a unit of emf. This value is called Volt, i.e. 1V=1.6·10-19 J/el. charge. The energy required to remove an electron or transfer it from one atom to another is approximately equal to 1 volt times the elementary charge. That is why the electron volt is used as a unit of energy measurement. The electron volt is useful for measuring the energy of "chemical reactions" between individual particles and the energies required to ionize individual atoms. Then, if we took 1B = 1.6·10-19 J/elem as a unit of energy. charge, then for a unit of charge it is necessary to introduce a new unit, for example a coulomb so that the product of one coulomb by an emf of one volt gives one joule 1 coulomb 1 V = 1 J, then 1 coulomb = 1 J / 1 V = 1.6 10- 19 J/elem. charge=6.25·1018 element. charges. With this choice of charge units, the current strength is ampere, so 1A = 6.25 1018 el. charges per second. In conductors of the first kind, the charge carriers are electrons, and in conductors of the second kind, they are ions. Current, as noted above, is the result of the interaction of electromagnetic energy IE with matter; it is a closed substance and represents a continuous flow of electric charge. Therefore, transformations occur at the boundaries of fields (conductors): at the anode, anions must give up extra electrons and turn into neutral atoms, and at the cathode, on the contrary, cations must receive electrons and turn into neutral atoms. Only in this case the total current in the electrolyte will be equal to the electronic current in the metal part of the circuit. However, this phenomenon is accompanied by chemical decomposition of the electrolyte and, therefore, the speed, electrical conductivity and other parameters in conductors of the second kind will depend on the degree of chemical decomposition (called dissociation) of the electrolyte, and the magnitude of the total electric field E + Estor [1].