Electric current in liquids
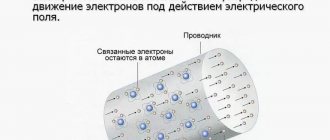
Like solids, liquids can be dielectrics and conductors. Distilled water, for example, is a dielectric, and a small amount of sodium chloride NaCl (also a dielectric) added to distilled water makes it a conductor.
This is explained as follows. In distilled water, the concentration of free charges is very small, so it conducts current poorly. The dielectric constant of water is ε = 81, therefore, when a substance is dissolved in water, the Coulomb forces of interaction between ions in the salt molecule decrease. And the energy of thermal (random) movement of particles may be enough for the molecule to break up into Na+ and Cl– ions.
- The breakdown of the molecules of a substance into ions when it is dissolved in a liquid is called electrolytic dissociation
.
The theory of electrolytic dissociation was developed in 1887 by the German scientist R. Clausius and the Swedish chemist S. Arrhenius.
Molecules of different substances dissociate differently and can split into two or more ions. The nature of dissociation is closely related to the chemical properties of the substance.
For example, when copper sulfate salt is dissolved in water, the CuSO4 molecule dissociates into two ions: Cu2+ and SO42-:
\(~CuSO_4 \leftrightarrows Cu^{2+} + SO_4^{2-}.\)
In the absence of an external electric field, the ions are in thermal chaotic motion.
When ions of opposite sign meet, they can again form a neutral molecule. This process is called recombination
ions (the reverse process of dissociation). Under constant conditions, a dynamic equilibrium is established in the solution, when the number of molecules that disintegrate into ions per second is equal to the number of pairs of ions that, at the same time, recombine into neutral molecules.
Degree of dissociation
α is determined by the ratio of the number of molecules disintegrated into ions to their total number. The degree of dissociation depends on temperature, solution concentration and dielectric constant of the solvent. Since the energy of thermal motion of molecules increases with increasing temperature, the degree of dissociation of the electrolyte increases and, consequently, the concentration of positively and negatively charged ions increases.
Let two electrodes, which are metal conductors, be placed in a vessel with an electrolyte solution, to which we connect an EMF source. The electrode connected to the positive terminal of the source is called the anode
, to the negative terminal -
cathode
.
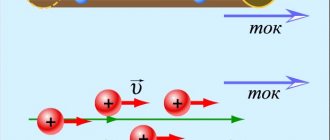
An electric field will arise in the vessel, and negative ions (anions) will begin to move towards the anode, and positive ions (cations) - towards the cathode (Fig. 1). As a result, an electric current will be established in the electrolyte solution. Rice. 1 The term “ion” is translated from Greek as “going.” This is where the names “ anion
” — going to the anode, and “
cation
” — going to the cathode — come from.
- Electric current in liquids
is the directed movement of ions of both signs.
Since charge transfer in electrolytes is carried out by ions, such conductivity is called ionic
.
However, some liquids can also exhibit electronic conductivity
. Liquid metals, for example, have such conductivity.
- Liquids that conduct electric current are called electrolytes
.
For electrolytes, Ohm's law and the Joule-Lenz law are also valid.
In ionic conduction, the passage of current is associated with the transfer of matter. At the electrodes, substances that make up the electrolytes are released. At the anode, negative ions give up their extra electrons (this is called an oxidation reaction), and at the cathode, positive ions receive the missing electrons (a reduction reaction). By giving or receiving electrons, ions become neutral atoms. These atoms (or molecules formed from them) are released on the electrodes.
The resulting atoms can react with the electrodes or solvent.
Chemical reactions into which neutralized ions enter are called secondary
.
- The phenomenon of the release of substances on the electrodes when an electric current passes through the electrolyte is called electrolysis
.
A necessary condition for electrolysis is the passage of a direct electric current through the electrolyte.
Electrolysis was first observed in 1800 by W. Nicholson and A. Carlyle, who decomposed water using direct current. After 7 years, G. Davy isolated and discovered sodium using electrolysis.
Lecture 2. Electrical conductivity of electrolytes
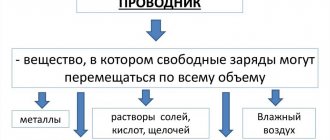
Substances whose aqueous solutions conduct electric current are called electrolytes. Unlike metals (electronic conductivity) or semiconductors (electron-hole conductivity), electrolytes have ionic conductivity .
Sometimes conductive solutions themselves are called electrolytes, although a more correct expression is an electrolyte solution.
Electrolytes are salts, acids, alkalis, etc., i.e. substances whose atoms in the molecules are connected by ionic (sometimes heteropolar covalent bonds).
When such substances are dissolved in water, the molecules dissociate into ions. The cause of electrolytic dissociation is the interaction of solute molecules with water molecules. The water molecule has a large dipole moment (p = 6.1 • 10-30 C • m, and ε -
81), therefore, at a distance of about 0.1 nm (the average intermolecular distance in a liquid) there is a fairly strong electric field around the water molecule. The latter is the direct cause that weakens the force of electrostatic interaction between ions in a dissolved molecule. The energy of interaction between ions in electrolyte molecules is close to the energy of interaction between the same ions and water molecules. Therefore, in the process of dissolving a salt or alkali, molecules disintegrate into ions due to thermal collisions.
Positive ions are called cations, negative ions are called anions .
The dissociation process is always reversible - along with dissociation, recombination of ions also takes place.
If the molecules of a solute in water do not dissociate into ions, then the solution is not a conductor. Aqueous solutions of sugars, glycerin, etc.
-
insulators.
The result of dissociation is the formation of solvates. when water molecules “envelop” ions, forming a solvation shell around them (Fig. 2.1).
Solvation has two important consequences:
1) the solvation shell prevents the recombination of ions, therefore at low concentrations the dissociation is complete:
2) the presence of a solvation shell complicates the movement of ions - it is not the ion that moves in the electric field, but the solvate; the charge of the solvate is less than the charge of the ion (screening effect of the solvation shell), and the dimensions are larger.
To generate an electric current in the electrolyte, it is necessary to lower electrodes made of a conductive material (metal, coal, etc.) into a bath with an electrolyte solution, to which a source of emf is connected (Fig. 2.1). Such a device is called a galvanic or electrolytic bath.
The processes occurring near the electrodes (at a distance of 1-10 molecular diameters) will differ significantly from the processes in the bulk of the solution.
Biological fluids are electrolytes. In these media, under the influence of an electric field, an ordered (directed) movement of free electric charges (electrons, ions or solvates) occurs - an electric current.
In the thickness of the solution, positive solvates will move towards the cathode at a speed, and negative solvates will move towards the anode at a speed.
The scalar characteristic of electric current is the current strength (I) , equal to the ratio of the charge (Dq) transferred through a cross section of a conductor or some surface over a time interval D t to
this interval:
(1)
If the electric current is uniformly distributed over the cross-section of the conductor, then the ratio of the current strength to the cross-sectional area of the conductor (S) is called current density (j) :
(2)
Let us establish a connection between current density and some characteristics of current carriers, molar concentration and speed of directional movement of particles. Let's write this formula for the particle flux density , replacing the molar concentration with concentration n :
(3)
If this formula is multiplied by the charge q of the current carrier, then the product qJ will correspond to the charge passing through a unit cross-sectional area in one second, i.e. will be the current density:
(4)
As can be seen, the current density is directly proportional to the charge of the current carrier, the concentration of carriers and the speed of their directional movement. Naturally, expression (4) is valid when the charges of the current carriers are equal and their speed is the same.
The current density for electrolytes should be presented as the sum of expressions for the current density for positive and negative ions, i.e. the total current density is equal to:
(5)
If we assume that each molecule dissociates into two ions, then the concentration of positive and negative ions is the same:
(6)
where α is the dissociation coefficient, n is the concentration of electrolyte molecules.
The directional movement of ions in an electric field can be approximately considered uniform, with the force qE ,
acting on the ion from the electric field is balanced by the friction force
rv
(7)
from where, replacing q/r
=
b ,
we get
(8)
The proportionality coefficient b is called the mobility of charge carriers (ions).
It is equal to the ratio of the speed of directional movement of ions caused by an electric field to the strength of this field. The mobility of charge carriers
b is related to the mobility of diffusing particles by the relation b
=
uq .
For ions of different signs from (8), we respectively have
. (9)
Substituting (6) and (9) into (5), we find
. (10)
Let us imagine the electrolyte in the form of a rectangular parallelepiped with electrode faces of area S ,
located at a distance
l (Fig. 2.2.).
Considering the field homogeneous, taking into account that , (11)
transform (10):
. (12)
Since I = jS,
then (12) corresponds to Ohm’s law for a section of the circuit without a current source: , where
(13)
- electrolyte resistance. Comparing with the relation, we get
. (14)
It follows that the specific conductivity of the electrolyte g is greater, the greater the concentration of ions, their charge and mobility .
As the temperature increases, the electrical conductivity of electrolytes increases, as the degree of dissociation and mobility of ions increases, the viscosity of the solution decreases and the electrical conductivity increases.
Near the surface of the electrode, more complex processes occur that are more electrochemical than purely physical:
a) electro-oxidation of anions occurs at the anode, electro-reduction of cations occurs at the cathode, and a number of other electrical processes also occur; in general, these processes are called polarization phenomena;
b) secondary chemical reactions can also occur near the surface of the electrodes.
At sufficiently low potentials, redox processes do not occur on the electrodes, therefore, for the galvanic bath as a whole, there are potential regions where the dependence of current on voltage does not obey Ohm’s law.
At sufficiently high potentials, the release of a substance on the electrodes may begin in the form of a sediment (deposition on the electrode) or gas. These processes are described quantitatively by Faraday's laws.
Faraday's first law : the mass of the substance released on the electrode is proportional to the electric charge flowing through the electrolyte:
(15)
where M is the mass of the substance, q is the charge, I is the current strength and t is time. Coefficient k , called the electrochemical equivalent of a substance, shows what mass of a substance will be released on the electrode when a charge equal to 1 C passes through the electrolyte.
Faraday's Second Law : The electrochemical equivalents of elements are directly proportional to their chemical equivalents:
(16)
where A is the atomic weight of the element; Z is its valency: A/Z is the chemical equivalent of the element.
The Faraday number F is numerically equal to the electric charge that must pass through the electrolyte in order for one kilogram equivalent of the substance to be released at the electrode. F = 9.6487×107 C/kg-eq.
As a result of the electroreduction or electrooxidation of electrolyte ions, electrically neutral atoms are formed on the electrodes, which will not necessarily be deposited on the electrodes or released in the form of gas bubbles - they can enter into chemical reactions with the solution near the electrode. Such processes will be secondary reactions.
All these processes are used in various branches of technology, many of them are also used in medicine
Laws of electrolysis
Electrolysis is described by two basic laws, experimentally established by Faraday in 1833-1834.
Faraday's first law
:
- the mass of substance m released on one of the electrodes is directly proportional to the charge Δ q
passed through the electrolyte:
m = K⋅Δq = K⋅I
⋅Δ
t
.
Here I
- current strength in the electrolyte, Δ
t
- time of current flow through the electrolyte,
K
- electrochemical equivalent of the substance.
- The electrochemical equivalent
is numerically equal to the mass of the substance
m
released on the electrode when a charge
q
of 1 C passes through the electrolyte solution. The SI unit of electrochemical equivalent is kilogram per coulomb (kg/C).
Table 1.
Electrochemical equivalents of substances
| Substance (anions) | K, 10–6 kg/Kl | Substance (cations) | K, 10–6 kg/Kl |
| Hydroxyl (OH–) | 0,177 | Aluminum (Al3+) | 0,0932 |
| Oxygen (O2–) | 0,0829 | Hydrogen (H+) | 0,1045 |
| Acid residue (SO42–) | 0,499 | Iron (Fe3+) | 0,193 |
| Sulfur (S2–) | 0,167 | Gold (Au3+) | 0,681 |
| Chlorine (Cl–) | 0,367 | Copper (Cu2+) | 0,329 |
| Sodium (Na+) | 0,238 | ||
| Nickel (Ni2+) | 0,30 | ||
| Silver (Ag+) | 1,11 | ||
| Mercury (Hg+) | 2,079 | ||
| Zinc (Zn2+) | 0,339 |
Faraday's second law:
- the electrochemical equivalent of a substance K
is directly proportional to its chemical equivalent:
\[K=C\cdot \dfrac{M}{Z} .\]
Here C
- proportionality coefficient, constant value, ratio $\dfrac{M}{Z} $ - chemical equivalent,
M
- molar mass of the substance,
Z
- valency of the substance.
This law is usually written in a different form, taking into account that $C = \dfrac{1}{F}$, where F
called Faraday's constant:
\[K=\frac{1}{F} \cdot \dfrac{M}{Z} .\]
- Faraday's constant F
is equal to the product of the elementary charge
e
and Avogadro's number
NA
:
F = e⋅NA
,
F
= 9.65⋅104 C/mol.
Faraday's laws played an important role in the history of the development of physics. They served as an impetus for putting forward the hypothesis about the existence of an elementary electric charge in nature and made it possible for the first time to determine its value.
See also
- Kikoin A.K. On the Faraday number and the specific charge of a charged particle // Quantum. - 1985. - No. 2. - P. 25-26
Current in electrolytes and gases. Ionization potential. Ohm's law for electrolytes and gases. Electrophoresis.
If two solid plates (electrodes) are introduced into an electrolyte or melt and a voltage is applied to them, an electric current arises, which is created by the directed movement of ions. Having reached the appropriate electrodes, the ions give up or gain electrons and turn into neutral atoms or molecules. As a result of chemical reactions, secondary products either settle on the electrodes or go into solution. The phenomenon of deposition of electrolyte components on electrodes is called electrolysis.
Materials in which chemical transformations occur during the passage of current are classified as conductors of the second type.
Electrolysis is quantitatively described by Faraday's laws:
, , (19)
where m is the mass of the substance deposited on the electrode, k is its electrochemical equivalent, i
= f(t) – current strength, t – time of its flow, F – Faraday number (F = 96.497∙10 6 C/mol.), M – molar mass of the substance, z – valence, F/z – is called the chemical equivalent of the substance .
If the current value I does not change during the electrolysis process, then (18) takes the form:
(20)
In solution, the processes of dissociation and recombination occur in parallel. Ultimately, dynamic equilibrium is established in the solution under constant external conditions. This state corresponds to a certain degree of dissociation, which is usually characterized by the dissociation coefficient - α, which shows the proportion of disintegrated molecules of the solute - α = n ' / n. At low temperatures, ions are surrounded by solvent ions clinging to them. This phenomenon is called solvation (for aqueous solutions - hydration), and the complex itself of an ion and a shell of solvent molecules held by its force field is called a solvate. Relationship
(21)
called ion mobility
which represents the average
drift speed of charged particles in a field with a strength of 1 V/m). = m 2 / (V s). The mobility of b ions depends on their nature, solvent properties and temperature. For steady motion, the current density in the electrolyte will be:
Application of electrolysis in technology
Electrolysis is widely used in technology.
Purification or refining of metals
. The process takes place in an electrolytic bath. The anode is the metal to be purified, the cathode is a thin plate of pure metal, and the electrolyte is a solution of a salt of a given metal, for example, when refining copper, a solution of copper sulfate. Contaminated metals may contain valuable impurities. Thus, copper often contains nickel and silver. In order for only pure metal to be released at the cathode, it must be taken into account that the release of each substance begins only at a certain potential difference between the electrodes, called the “decomposition potential”. With proper selection, pure copper is released from the copper sulfate solution at the cathode, and impurities precipitate or pass into solution.
Electrometallurgy
. Some metals, such as aluminum, are produced by electrolysis from molten ore. An iron box with a carbon floor serves as an electrolytic bath and at the same time a cathode, and carbon rods serve as an anode. The temperature of the ore (about 900 °C) is maintained by the current flowing through it. Molten aluminum falls to the bottom of the box, from where it is released through a special hole into molds for casting.
Galvanostegy
- an electrolytic method of coating metal products with a layer of noble or other metal (gold, platinum) that cannot be oxidized. For example, when nickeling an object, it itself serves as a cathode, and a piece of nickel serves as an anode. By passing electric current through the electrolytic bath for some time, the object is coated with a layer of nickel of the required thickness.
Electrotype
, or the electrolytic deposition of metal on the surface of an object to reproduce its shape, was invented in 1837 by the Russian scientist B. S. Jacobi, who proposed using electrolysis to obtain metal prints of relief objects (medals, coins, etc.). A wax cast is taken from the object or a convex image is cut out on a wooden board and made conductive by covering it with a layer of graphite. The cast or board is then dipped into the electrolyte as a cathode. The anode is a piece of metal used for deposition. This method is used to make, for example, printing clichés.
Heavy water (D2O) is produced electrolytically, in which the hydrogen atoms are replaced by atoms of its isotope, deuterium (D) with an atomic mass of 2.
See also
- Myakishev G.Ya. Physics: Electrodynamics //§3.6. Technical Application of Electrolysis
What substances are electrolytes? Electrolysis
Positively and negatively charged ions are carriers of free charges in electrolytes. Compounds of metals in a molten state, some solids are classified as electrolytes. Their main representatives are aqueous solutions of inorganic acids, salts, and bases. They are widely used in technology.
When an electric current passes through the electrolyte, substances are simultaneously released on the electrodes. This phenomenon is called electrolysis.
Electric current in electrolytes is considered as the movement of ions with both signs in opposite directions.
The movement of positive ions is directed towards the negative electrode (cathode), and negative ions - towards the positive electrode (anode). The appearance of ions with opposite signs in aqueous solutions of salts, acids, and alkalis is a consequence of the splitting of neutral molecules. The phenomenon is called electrolytic dissociation .
When copper chloride CuCl 2 dissociates into copper and chlorine ions in an aqueous solution, we obtain the expression:
CuCl 2 ⇄ Cu 2 + + 2 Cl - .
Picture 1 . 15 . Figure 1 shows the principle of the ordered movement of positive copper ions to the cathode, and negative chlorine ions to the anode due to the connection of the electrodes to a current source under the action of an electric field on these ions.
After reaching the cathode, the copper ions are neutralized by the excess number of its electrons and become neutral atoms, deposited on the cathode. Chlorine ions, having reached the anode, give up one electron each. After this, neutral chlorine atoms combine in pairs to form a chlorine molecule Cl 2 . Its presence is due to the release of bubbles at the anode.
Many electrolysis reactions are accompanied by secondary reactions of decomposition products that are released at the electrodes, with its material or solvents.
An example is the electrolysis of a solution of copper sulfate (copper sulfate) CuSO 4 with electrodes made of copper immersed in the electrolyte.
The dissociation of copper sulfate molecules proceeds according to the formula:
CuSO 4 ⇄ Cu 2 + + SO 4 2 - .
Neutral copper atoms are deposited as a solid deposit on the cathode. This produces chemically pure copper. When the SO 4 2 ion gives up two electrons to the anode, it becomes a neutral SO 4 radical, which enters into a secondary reaction with the copper anode:
SO 4 + Cu = CuSO 4.
The resulting copper sulfate molecule goes into solution. This shows the passage of electric current through an aqueous solution of copper sulfate to dissolve the copper anode and deposit copper on the cathode. The concentration of this solution does not change.
Picture 1 . 15 . 1 . Electrolysis of an aqueous solution of copper chloride.
Literature
- Aksenovich L. A. Physics in secondary school: Theory. Tasks. Tests: Textbook. allowance for institutions providing general education. environment, education / L. A. Aksenovich, N. N. Rakina, K. S. Farino; Ed. K. S. Farino. - Mn.: Adukatsiya i vyakhavanne, 2004. - P. 282-287.
- Burov L.I., Strelchenya V.M. Physics from A to Z: for students, applicants, tutors. - Mn.: Paradox, 2000. - P. 228-232.
- Zhilko, V.V. Physics: textbook. allowance for 11th grade. general education school from Russian language training / V.V. Zhilko, A.V. Lavrinenko, L. G. Markovich. — Mn.: Nar. Asveta, 2002. - pp. 258-263.