Thermal conductivity and density of aluminum
The table shows the thermophysical properties of aluminum Al depending on temperature.
The properties of aluminum are given over a wide temperature range - from minus 223 to 1527 ° C (from 50 to 1800 K). As can be seen from the table, the thermal conductivity of aluminum at room temperature is about 236 W/(m deg), which allows this material to be used for the manufacture of radiators and various heat sinks.
In addition to aluminum, copper also has high thermal conductivity. Which metal has the greater thermal conductivity? It is known that the thermal conductivity of aluminum at medium and high temperatures is still less than that of copper, however, when cooled to 50K, the thermal conductivity of aluminum increases significantly and reaches a value of 1350 W/(m deg). For copper, at such a low temperature, the thermal conductivity value becomes lower than for aluminum and amounts to 1250 W/(m deg).
Aluminum begins to melt at a temperature of 933.61 K (about 660 ° C), while some of its properties undergo significant changes. The values of properties such as thermal diffusivity, aluminum density and thermal conductivity are significantly reduced.
The density of aluminum is mainly determined by its temperature and depends on the state of aggregation of this metal. For example, at a temperature of 27°C, the density of aluminum is 2697 kg/m3, and when this metal is heated to its melting point (660°C), its density becomes equal to 2368 kg/m3. The decrease in aluminum density with increasing temperature is due to its expansion when heated.
Receipt
Aluminum forms a strong chemical bond with oxygen. Compared to other metals, the reduction of aluminum to metal from natural oxides and aluminosilicates is more difficult due to its high reactivity and the high melting point of all its ores, such as bauxite and corundum.
Conventional reduction to metal by firing the oxide with carbon (as, for example, in metallurgical processes for the reduction of iron) is impossible, since aluminum has a higher affinity for oxygen than carbon.
It is possible to obtain aluminum through incomplete reduction of aluminum with the formation of an intermediate product - aluminum carbide Al4C3, which then undergoes decomposition at 1900-2000 ° C to form metallic aluminum. This method of producing aluminum is being studied and it is believed that it is more profitable than the classical electrolytic method of producing aluminum, the Hall-Heroult process, since it requires less energy and leads to the formation of less CO2.
The modern production method, the Hall-Héroult process, was developed independently by the American Charles Hall and the Frenchman Paul Héroult in 1886. It consists of dissolving aluminum oxide Al2O3 in molten cryolite Na3AlF6, followed by electrolysis using consumable coke or graphite anode electrodes. This production method requires very large amounts of electricity and therefore received industrial application only in the 20th century.
Electrolysis in molten cryolite:
2Al2O3 →Na3AlF6 4Al + 3O2
To produce 1000 kg of crude aluminum, 1920 kg of alumina, 65 kg of cryolite, 35 kg of aluminum fluoride, 600 kg of anode graphite electrodes and about 17 MWh of electricity (~61 GJ) are required.
A laboratory method for producing aluminum was proposed by Friedrich Wöhler in 1827 by reducing anhydrous aluminum chloride with potassium metal (the reaction occurs when heated without access to air):
AlCl3 + 3K → 3KCl + Al
Conductivity - Aluminum
Aluminum has high thermal and electrical conductivity. The electrical conductivity of aluminum also depends on its purity.
And the electrical conductivity of aluminum is only one third lower than the electrical conductivity of copper. These circumstances and the fact that aluminum has become significantly cheaper than copper (in our days - about 2 5 times) were the reason for the massive use of aluminum in wires and in electrical engineering in general.
Choosing a flux for soldering aluminum
In the form of pure metal, aluminum is used for the manufacture of chemical equipment, electrical wires, and condensates. Although the electrical conductivity of aluminum is less than that of copper (about 0% of the electrical conductivity of copper), this is compensated by the lightness of aluminum, which allows the wires to be made thicker: with the same electrical conductivity, an aluminum wire weighs half as much as a copper wire.
In the form of pure metal, aluminum is used for the manufacture of chemical equipment, electrical wires, and capacitors. Although the electrical conductivity of aluminum is less than that of copper (about 60% of the electrical conductivity of copper), this is compensated by the lightness of aluminum, which allows the wires to be made thicker: with the same electrical conductivity, an aluminum wire weighs half as much as a copper wire.
In the form of pure metal, aluminum is used for the manufacture of chemical equipment, electrical wires, and capacitors. Although the electrical conductivity of aluminum is only about 60% of the electrical conductivity of copper, this is compensated by the lightness of aluminum, which allows the wires to be made thicker: with the same electrical conductivity, an aluminum wire weighs half as much as a copper wire.
Pure aluminum partially replaces copper in electrical engineering; It is used to make wires, buses, contacts and other products that must have high electrical conductivity. Although the electrical conductivity of aluminum is only 65% of the electrical conductivity of copper, its density is more than three times lower, and therefore the metal consumption is lower in weight terms.
In the form of pure metal, aluminum is used for the manufacture of chemical equipment, electrical wires, and capacitors. Although the electrical conductivity of aluminum is less than that of copper (about 60% of the electrical conductivity of copper), this is compensated by the lightness of aluminum, which allows the wires to be made thicker: with the same electrical conductivity, an aluminum wire weighs half as much as a copper wire.
More important is the ratio Z) / (S 28n), where 5P is the depth of penetration of the magnetic flux into the slot space in the transition mode. An increase in the electrical conductivity of aluminum due to its cooling reduces the depth of penetration of the magnetic flux into the body of the screen and thereby increases the degree of transverse compression of the magnetic flux. The highest degree of compression is provided by superconducting screens.
Pure aluminum is used in electrical engineering for the manufacture of current conductors. The heat and electrical conductivity of aluminum is slightly lower than that of pure copper. All impurities present in aluminum impair its heat and electrical conductivity.
A more noticeable effect is exerted by impurities of copper, silver and magnesium, which reduce the electrical conductivity of aluminum by 5 - 10% at the same weight content. Additions of titanium and manganese greatly reduce the electrical conductivity of aluminum.
A more noticeable effect is exerted by impurities of copper, silver and magnesium, which reduce the electrical conductivity of aluminum by 5 - 10% at the same weight content. Additions of titanium and manganese greatly reduce the electrical conductivity of aluminum.
- Names and composition of copper alloys
According to VDE 0202 / VII.43, the change in resistance of an aluminum conductor for electrical purposes, having a length of 1 m and a cross-sectional area of 1 mm2, is 1 1 10 - 4 ohm / C. With the lowest electrical conductivity of aluminum used for conductors, x 36 this corresponds to the critical temperature f 0 232 C. According to measurements made by the author, for aluminum, which has such electrical conductivity, the value of the critical temperature turns out to be slightly higher. GOST 183 - 55 recommends for aluminum up to 0 245 C.
It conducts heat and electricity well. Depending on the purity, the electrical conductivity of aluminum is 62 - 65 / and the electrical conductivity of copper.
When welding Ml copper with A5 grade aluminum over a layer of standard flux used for welding aluminum (AN-A1) with a metal thickness of up to 20 mm, use AD1 grade wire with a diameter of 2-5 mm. MPa, the electrical conductivity remains at the level of the electrical conductivity of aluminum.
Aluminum is also noticeably inferior to copper in its ability to conduct electric current. Unlike copper, annealing does not change the electrical conductivity of aluminum.
Thermal conductivity - aluminum - Great Encyclopedia of Oil and Gas, article, page 2
Thermal Conductivity - Aluminum
Page 2
The strength of the aluminum shell is several times higher than that of lead, aluminum is 4 2 times lighter than lead (specific gravity 2 7 and 11 4, respectively), the thermal conductivity of aluminum is approximately six times higher than that of lead, its resistance to vibration fatigue is 25 times greater than at lead. In four-wire AC networks with voltages up to 1000 V with a solidly grounded neutral, it is allowed to use an aluminum sheath as a neutral working wire. [16]
In this equation, di 15 5 - 10 - 3 ( m) is the outer diameter of the graphite cylinder; d0 1 1 45 - 10 - 3 ( m) - cross-sectional diameter of the molten metal being tested; q ( z) ( kcal / m2 - hour) - heat flow on the outer surface of the graphite cylinder; K AI and gr (kcal / m - hour - deg) are the thermal conductivity coefficients of aluminum and graphite, respectively. [17]
The best metals to conduct heat are silver and copper. The thermal conductivity of aluminum is approximately 2 5 times, iron 1 time, lead 12 times less than copper. [18]
The corrosive effect of some flux components on aluminum is neutralized by washing the seam and surfaces of parts with a 10% solution of nitric acid in warm water and subsequently with hot water. The thermal conductivity of aluminum is almost 5 times, and the heat capacity is 2 times greater than steel, so when welding aluminum it is necessary to maintain a higher flame temperature than the melting point of aluminum. [19]
The thermal conductivity of aluminum is 3 times greater than that of steel, the expansion coefficient is 2 times higher than the expansion coefficient of steel. [21]
The crystal lattice of aluminum consists, like many other metals, of face-centered cubes (see page. The thermal conductivity of aluminum is twice the thermal conductivity of iron and equal to half the thermal conductivity of copper. Its electrical conductivity is much higher than the electrical conductivity of iron and reaches 60% of the electrical conductivity of copper. [22]
The best metals to conduct heat are silver and copper. The thermal conductivity of aluminum is approximately 2 5 times less, that of iron is 6 times less, that of lead is 12 times less, and that of copper. [23]
As the purity of aluminum decreases, thermal conductivity decreases, and with increasing temperature it increases slightly. At 100, the thermal conductivity of aluminum is 66 5% of the thermal conductivity of silver. [24]
If this amount of heat is known, then for section z, from the measured value of the temperature gradient in it, it is possible to calculate the value of the thermal conductivity coefficient of the sample. The final calculation of the desired value of the thermal conductivity coefficient of aluminum consists of calculating the correction for the thermal conductivity coefficient of the sample for the heat passing through the walls of the graphite cylinder. [25]
The atomic structure of titanium and its high electron affinity have a strong influence on properties such as electrical and thermal conductivity. Its thermal conductivity is 8 - 10 times less than the thermal conductivity of aluminum. This is of significant importance, for example, when cutting metal. [27]
The modulus of elasticity of titanium is almost half that of iron, is on the same level as that of copper alloys and is significantly higher than that of aluminum. The thermal conductivity of titanium is low: it is about 7% of the thermal conductivity of aluminum and 16-5% of the thermal conductivity of iron. This must be taken into account when heating metal for pressure treatment and welding. The electrical resistance of titanium is approximately 6 times greater than that of iron and 20 times greater than that of aluminum. [28]
The modulus of elasticity of titanium is almost half that of iron, is on the same level as that of copper alloys and is significantly higher than that of aluminum. The thermal conductivity of titanium is low: it is about 7% of the thermal conductivity of aluminum and 16-5% of the thermal conductivity of iron. [29]
Fiberglass based on phenolic resins have thermal conductivity of the same order. For comparison, it should be noted that the thermal conductivity of steel is 40, and the thermal conductivity of aluminum ranges from 175 to 200 kcal/m-h-deg. [thirty]
Pages: 1 2 3 4
www.ngpedia.ru
Why aluminum
Although aluminum conductors do not have high performance characteristics, they do:
- cheap compared to copper;
- are light in weight. Thus, aluminum wire is 3 times lighter than copper;
- universal in use - the operating temperature range is quite wide from –50 ⁰С to +50 ⁰С;
- resistant to high humidity - up to 98%;
- resistant to corrosion damage. Although there are nuances here too: the surface of any aluminum product in air is instantly susceptible to oxidation and is immediately covered with a film that protects the wire from further oxidation.
It would seem that it is more profitable to use aluminum products than copper ones. But she also has a number of negative qualities. Thus, the disadvantages of aluminum conductors are the low mechanical strength of the material, and the connection of such wires causes problems in the passage of current through them. Besides:
- the conductivity of aluminum is not high enough - 0.0271 Ohm×mm²/m;
- aluminum is susceptible to oxidation, and its film, which appears after it, does not conduct electricity well. But here, too, there is a catch: this film consists of particles of the upper layer of the conductor itself, which separates from the overall structure and thereby reduces its diameter. As a result, the initial resistance characteristic of aluminum wire increases;
- Due to the increased resistance of the film on aluminum wiring in the places where its individual parts are connected, the contact resistance increases, which causes the wiring to heat up. Therefore, if the service life of the aluminum wires used is exceeded, this may lead to a fire;
- Aluminum is not elastic and very brittle. Moreover, fragility increases after overheating.
Whether to use aluminum or copper wires depends on the tasks and priorities.
Thermal conductivity - aluminum - Great Encyclopedia of Oil and Gas, article, page 1
Thermal Conductivity - Aluminum
Page 1
The thermal conductivity of aluminum is more than 3 times higher than the thermal conductivity of iron, which leads to strong heat removal and a wide heating zone of the metal adjacent to the seam. [1]
The thermal conductivity of aluminum is five times greater than that of cast iron, and therefore aluminum alloys often replace cast iron in the manufacture of pistons for internal combustion engines. In addition, an aluminum alloy piston, being approximately three times lighter than a cast iron one, lightens the weight of the structure. Metals with high thermal conductivity are at the same time the best conductors of electricity. [2]
The high heat capacity and thermal conductivity of aluminum ensure uniform temperature along the entire length of the tube. [4]
Since the thermal conductivity of aluminum is almost five times higher than the thermal conductivity of steel, the heating time, and therefore the vulcanization time of rubber compounds in molds made of this material, is reduced. However, it should be noted that aluminum molds wear out quickly, which is their significant drawback. [5]
Impurities have a significant effect on the thermal conductivity of aluminum at low temperatures. [7]
The thermal conductivity of the oxide film is much worse than the thermal conductivity of aluminum, but due to the insignificant thickness of the film, this does not have a noticeable effect on the overall thermal conductivity of the product. [8]
Titanium has low thermal conductivity, which is 13 times less than the thermal conductivity of aluminum and 4 times less than the thermal conductivity of iron. With increasing temperature, the thermal conductivity of titanium decreases slightly and at 700 C is 0.0309 cal/cm sec CC. [9]
Titanium has low thermal conductivity, which is 13 times less than the thermal conductivity of aluminum and 4 times less than the thermal conductivity of iron. With increasing temperature, the thermal conductivity of titanium decreases slightly and at 700 C is 0.0309 cal/cm sec C. [10]
Therefore, for example, the thermal conductivity of titanium is 8–10 times less than the thermal conductivity of aluminum. [eleven]
The thermal conductivity coefficient of copper, silver and steel changes slightly with temperature, the thermal conductivity of aluminum increases in the range 0 - 400 C approximately 1 6 times. At high temperatures, silver evaporates more intensely than copper, and copper oxidizes and interacts with tellurides vapors. Therefore, for copper busbars it is advisable to use protection with a layer of iron. The contact of the tires with thermoelements is carried out through intermediate layers, which prevent the diffusion of the tire material into the thermoelectric material. [12]
Therefore, for example, the thermal conductivity of titanium is 8–10 times less than the thermal conductivity of aluminum. [13]
From a comparison of the given data for aluminum with the thermophysical characteristics of alkali metals, it follows that the boiling point and thermal conductivity of aluminum are much higher, and the thermal neutron capture cross section is much smaller than the corresponding values for alkali metals. Bearing in mind that the remaining thermophysical characteristics of the compared metals are approximately the same, and also taking into account the low elasticity of aluminum vapor at high temperatures, we can conclude that from the point of view of the thermophysical characteristics, aluminum, as a coolant, has certain advantages over alkaline materials. metals when solving problems associated with high coolant temperatures. [14]
It should be emphasized that since the actual transient electrical resistance of the welding points (RK) is very small (it is measured in fractions of a microhm), and the thermal conductivity of aluminum and copper is high, overheating never occurs at the welding site when current passes, even in cases where the total The cross-section of the weld points is significantly smaller than the working cross-section of the tire itself. This has been thoroughly verified through extensive laboratory and field testing. [15]
Pages: 1 2 3 4
www.ngpedia.ru
Connection of copper and aluminum wires
Recently, electrical equipment of increasingly higher power has begun to be used in everyday life and industry. During Soviet times, wiring was made mainly of cheap aluminum. Unfortunately, its performance characteristics no longer meet the new requirements. Therefore, today in everyday life and in industry very often aluminum wires are replaced with copper ones. The main advantage of the latter, in addition to refractoriness, is that during the oxidation process their conductive properties do not decrease.
Nichrome
Often when modernizing electrical networks, aluminum and copper wires have to be connected. This cannot be done directly. Actually, the electrical conductivity of aluminum and copper does not differ too much. But only for these metals themselves. The oxidizing films of aluminum and copper have different properties. Because of this, the conductivity at the junction is significantly reduced. The oxidation film of aluminum has much greater resistance than that of copper. Therefore, the connection of these two types of conductors must be made exclusively through special adapters. These could be, for example, clamps containing a paste that protects metals from the appearance of oxide. This adapter option is usually used outdoors. Branch compressors are more often used indoors. Their design includes a special plate that eliminates direct contact between aluminum and copper. If such conductors are not available at home, instead of twisting the wires directly, it is recommended to use a washer and nut as an intermediate “bridge”.
Pharmaceuticals
Speaking about the versatility of aluminum, we cannot ignore an important fact: the metal from which dishes and airplanes are made is widely used to treat and prevent serious illnesses and is approved for these purposes by the World Health Organization. Of course, we are not talking about aluminum in its pure form, but about its compounds.
In 1926, it was discovered that diphtheria toxoid precipitated with alum (neutralized bacterial toxin) stimulates the production of antibodies much better than it in its pure form. Since then, aluminum salts have been most often used to enhance the effect of vaccines, since they are considered harmless to humans.
It is on the basis of aluminum that the most effective antacids are produced. Aluminum hydroxide, which neutralizes acid well, is needed to treat peptic ulcers, dyspepsia, and stomach irritation. Aluminum phosphate is suitable for the same purposes.
| Figure 10 - Medicines | Figure 11 - Deodorants |
But even those who have excellent health will benefit from a product containing aluminum, which is sold in any pharmacy, and not only that. We are talking about an antiperspirant deodorant. Even the ancient Greeks and Romans used alum to suppress secretion. Our grandmothers also used ordinary alum. Aluminum chloride was added to the first factory-made anti-sweat products, and the main agent in modern products is aluminum chlorohydrate. By the way, what the effect of their action is based on is still not known exactly [6].
Resistivity
this affects power loss
- Boards installed in computers are equipped with etched copper traces.
- Copper is also used to make a wide variety of components used in electronic devices.
- In transformers and electric motors it is represented by a winding, which is made of this material.
There is no doubt that the expansion of the scope of application of this material will occur with the further development of technological progress. Although there are other materials besides copper, designers still use copper when creating equipment and various installations. The main reason for the demand for this material is the good electrical and thermal conductivity of this metal, which it provides at room temperature.
Thermal conductivity of copper - how does it affect the properties of copper? + Video
The high thermal conductivity of copper, along with other remarkable properties, has given this metal a significant place in the history of the development of human civilization. Products made from copper and its alloys are used in almost all areas of our lives.
1 Copper – briefly about thermal conductivity
Thermal conductivity is the process of transferring the energy of particles (electrons, atoms, molecules) of more heated parts of the body to particles of its less heated parts. This heat exchange leads to temperature equalization. Only energy is transferred along the body, matter does not move. A characteristic of the ability to conduct heat is the thermal conductivity coefficient, numerically equal to the amount of heat that passes through a material with an area of 1 m2, a thickness of 1 m, in 1 second with a unit temperature gradient.
We recommend that you read
The thermal conductivity coefficient of copper at a temperature of 20–100 °C is 394 W/(m * K) - only silver is higher. Rolled steel is inferior to copper in this indicator by almost 9 times, and iron - by 6. Various impurities have different effects on the physical properties of metals. For copper, the rate of heat transfer decreases when substances such as:
- aluminum;
- iron;
- oxygen;
- arsenic;
- antimony;
- sulfur;
- selenium;
- phosphorus.
High thermal conductivity is characterized by the rapid spread of heating energy throughout the entire volume of the object. This ability has provided copper with widespread use in any heat transfer systems. It is used in the manufacture of tubes and radiators of refrigerators, air conditioners, vacuum units, and cars to remove excess heat from the coolant. In heating appliances, similar copper products are used for heating.
Copper's ability to conduct heat decreases when heated. The values of the thermal conductivity coefficient of copper in air depend on the temperature of the latter, which affects heat transfer (cooling). The higher the ambient temperature, the slower the metal cools and the lower its thermal conductivity. Therefore, all heat exchangers use forced airflow by a fan - this increases the efficiency of the devices and at the same time maintains thermal conductivity at an optimal level.
2 Thermal conductivity of aluminum and copper - which metal is better?
The thermal conductivity of aluminum and copper is different - for the first it is 1.5 times less than for the second. For aluminum, this parameter is 202–236 W/(m * K) and is quite high compared to other metals, but lower than that of gold, copper, and silver. The scope of application of aluminum and copper, where high thermal conductivity is required, depends on a number of other properties of these materials.
Aluminum is not inferior to copper in anti-corrosion properties and is superior in the following indicators:
- the density (specific gravity) of aluminum is 3 times less;
- the cost is 3.5 times lower.
A similar product, but made of aluminum, is much lighter than copper. Since the weight of metal required is 3 times less, and its price is 3.5 times lower, an aluminum part can be about 10 times cheaper. Thanks to this and its high thermal conductivity, aluminum is widely used in the production of tableware and food foil for ovens. Since this metal is soft, it is not used in its pure form - its alloys are mainly common (the most famous is duralumin).
In various heat exchangers, the main thing is the rate of release of excess energy into the environment. This problem is solved by intensively blowing the radiator using a fan. At the same time, the lower thermal conductivity of aluminum practically does not affect the quality of cooling, and equipment and devices are much lighter and cheaper (for example, computers and household appliances). Recently, there has been a tendency in production to replace copper tubes in air conditioning systems with aluminum ones.
Copper is practically irreplaceable in the radio industry and electronics as a conductive material. Due to its high ductility, it can be used to draw wire with a diameter of up to 0.005 mm and to make other very thin conductive connections used for electronic devices. Higher conductivity than aluminum ensures minimal losses and less heating of radio elements. Thermal conductivity allows you to effectively remove the heat generated during operation to the external elements of devices - housing, supply contacts (for example, microcircuits, modern microprocessors).
Copper templates are used in welding when it is necessary to deposit the desired shape on a steel part. High thermal conductivity will not allow the copper template to connect to the welded metal. Aluminum cannot be used in such cases, since there is a high probability of it melting or burning. Copper is also used in carbon arc welding - a rod made of this material serves as a non-consumable cathode.
3 Disadvantages of high thermal conductivity
Low thermal conductivity in many cases is a necessary property - thermal insulation is based on this. The use of copper pipes in heating systems leads to much greater heat losses than when using mains and wiring made of other materials. Copper pipelines require more careful thermal insulation.
Copper has high thermal conductivity, which causes a rather complex process of installation and other work that has its own specifics. Welding, soldering, and cutting copper requires more concentrated heating than for steel, and often preliminary and concomitant heating of the metal.
When gas welding copper, it is necessary to use torches with a power 1–2 numbers higher than for steel parts of the same thickness. If the copper is thicker than 8–10 mm, it is recommended to work with two or even three torches (welding is often carried out with one, and heating is carried out with the others). Welding work using alternating current with electrodes is accompanied by increased metal spattering. A cutter sufficient for a thickness of high-chromium steel of 300 mm is suitable for cutting brass, bronze (copper alloys) up to 150 mm thick, and pure copper only 50 mm thick. All work involves significantly higher costs for consumables.
4 How to increase the thermal conductivity of copper?
Copper is one of the main components in electronics and is used in all microcircuits. It removes and dissipates the heat generated by the passage of current. The speed limit of computers is due to the increase in heating of the processor and other circuit elements as the clock frequency increases. Splitting into several cores working simultaneously and other methods of combating overheating have exhausted themselves. Currently, developments are underway aimed at obtaining conductors with higher electrical and thermal conductivity.
Graphene, recently discovered by scientists, can significantly increase the thermal conductivity of copper conductors and their ability to dissipate heat. During the experiment, a layer of copper was coated with graphene on all sides. This improved the heat transfer of the conductor by 25%. As the scientists explained, the new substance changes the structure of heat transfer and allows energy to move more freely in the metal. The invention is being finalized - the experiment used a much larger copper conductor than in the processor.
tutmet.ru
Specific electrical conductivity of some substances (table)
Specific conductivity is given at +20 °C:
| Substance | Sm/m | Substance | Sm/m | Substance | Sm/m | Substance | Sm/m | Substance | Sm/m |
| silver | 62 500 000 | molybdenum | 18 500 000 | tin | 8 330 000 | mercury | 1 040 000 | marble | 10−8 |
| copper | 59 500 000 | tungsten | 18 200 000 | cast steel | 7 690 000 | nichrome | 893 000 | glass | 10−11 |
| gold | 45 500 000 | zinc | 16 900 000 | lead | 4 810 000 | graphite | 125 000 | porcelain | 10−14 |
| aluminum | 38 000 000 | nickel | 11 500 000 | nickel silver | 3 030 000 | sea water | 3 | quartz glass | 10−16 |
| magnesium | 22 700 000 | pure iron | 10 000 000 | constantan | 2 000 000 | the ground is wet | 10−2 | amber | 10−18 |
| iridium | 21 100 000 | platinum | 9 350 000 | manganin | 2 330 000 | distilled water | 10−4 |
Chemical properties
In terms of chemical properties, aluminum is one of the highly active metals with amphoteric properties. In the activity series it ranks behind the alkaline earth metals. But in its pure form, both in air and in water, it can be stored for a very long time, since its surface is eventually covered with a thin and very durable layer of oxide, which protects it from oxidation.
In order to observe the oxidation of aluminum in air, you must first remove the protective film.
To do this, aluminum is first rubbed with sandpaper and then boiled in alkali. Aluminum oxide, like the metal itself, exhibits amphoteric properties and therefore dissolves in alkali. After this, the aluminum is dipped into a solution of some mercury salt, for example, mercuric nitrate Hg(NO3)2. Aluminum, as a more active metal, displaces mercury from its salt:
2Al + 3Hg(NO3)2 = 2Al(NO3)3 + 3Hg
Mercury is deposited on the surface of aluminum, forming an alloy of aluminum with mercury - aluminum “amalgam” (alloys of mercury with metals are called amalgams). Such an alloy is not capable of forming a protective oxide film, and the aluminum in the amalgam is gradually oxidized to aluminum oxide according to the equation:
4Al + 3O2 = 2Al2O3
But since the amalgam covers the aluminum unevenly, oxidation occurs in places and aluminum oxide is noticeable on the surface of the metal in the form of a fluffy brush (Fig. 3).
The interaction of aluminum with halogens - with bromine and iodine - is interesting. For the reaction, powdered aluminum and liquid bromine are used, and for the reaction with iodine, a mixture of iodine powder and aluminum is used.
In all cases, aluminum behaves as a reducing agent.
Rice. 3. Formation of aluminum oxide on the amalgamated metal surface.
At high temperatures, aluminum displaces some metals from their oxides. This property has found application. If you mix iron oxide with aluminum powder and set it on fire using a magnesium flash, the reaction will occur:
Fe2O3 + 2Al = Al2O3 + 2Fe
which is accompanied by the release of a large amount of heat. Due to this heat, the resulting free iron melts and can be released from the crucible in which the reaction takes place through the hole located below. This smelting of metals is called aluminothermy; in technology it is used very widely. Some metals can only be produced by aluminothermic processes. This process was first carried out by N. N. Beketov.
Aluminum is an amphoteric metal. It behaves differently in different conditions. In an alkali solution, aluminum displaces hydrogen from water, forming a salt of aluminum acid - sodium (or potassium) aluminate, in which it plays the role of an acid-forming element:
Aluminum displaces hydrogen from acid:
In this case, it exhibits metallic properties. Concentrated nitric and sulfuric acids do not affect aluminum, since a protective film is formed on its surface, protecting the metal from further oxidation. In diluted form, nitric acid also has no effect on aluminum, and sulfuric acid has a weak effect.
■ 77. List the chemical properties of aluminum and justify your answer with reaction equations. (See Answer) 78. Why is mercury called “aluminum poison”? 79. Why do household aluminum products last a long time and are not subject to oxidation? 80. What is aluminothermy? 81. The dry mixture consists of aluminum, iron and coal powders. When 6 g of this mixture was treated with hydrochloric acid, 4.48 liters of hydrogen were released, and when the same amount of the mixture was treated with potassium hydroxide solution, 3.36 liters of hydrogen were released. Determine the composition of the mixture in grams. 82. There are 200 g of pyrolusite containing 87% manganese dioxide. How much aluminum will be required to recover manganese from it by aluminothermic means. 83. How should aluminum be treated so that it oxidizes in air? 84. Three test tubes contain dilute acids - hydrochloric, sulfuric and nitric. How, given pieces of aluminum, can you determine which test tube contains which acid? 85. How much sodium aluminate will be obtained when 27 g of aluminum reacts with alkali? (See answer)
Being in nature
Prevalence
In terms of prevalence in the earth's crust, it ranks 1st among metals and 3rd among elements, second only to oxygen and silicon. The mass concentration of aluminum in the earth's crust, according to various researchers, is estimated from 7.45 to 8.14%.
Natural aluminum compounds
In nature, aluminum, due to its high chemical activity, is found almost exclusively in the form of compounds. Some of the natural minerals of aluminum are:
- Bauxite – Al2O3 H2O (with admixtures of SiO2, Fe2O3, CaCO3)
- Nephelines - KNa3[AlSiO4]4
- Alunites - (Na,K)2SO4 Al2(SO4)3 4Al(OH)3
- Alumina (mixtures of kaolins with sand SiO2, limestone CaCO3, magnesite MgCO3)
- Corundum (sapphire, ruby, emery) – Al2O3
- Feldspars - (K,Na)2O Al2O3 6SiO2, Ca[Al2Si2O8]
- Kaolinite - Al2O3 2SiO2 2H2O
- Beryl (emerald, aquamarine) - 3BeO Al2O3 6SiO2
- Chrysoberyl (Alexandrite) - BeAl2O4.
However, in some specific reducing conditions (volcano vents) negligible amounts of native metallic aluminum have been found.
Natural waters contain aluminum in the form of low-toxic chemical compounds, for example, aluminum fluoride. The type of cation or anion depends, first of all, on the acidity of the aqueous medium. Aluminum concentrations in Russian water bodies range from 0.001 to 10 mg/l. In sea water its concentration is 0.01 mg/l.
Isotopes of aluminum
Main article: Isotopes of aluminum
Natural aluminum consists almost entirely of a single stable isotope, 27Al, with negligible traces of 26Al, the longest-lived radioactive isotope with a half-life of 720 thousand years, formed in the atmosphere when argon nuclei are split by 40Ar protons from high-energy cosmic rays.
Rolled and low-grade material
Electrical aluminum obtained during foundry production is accepted separately. Usually these are components for machine tools, automobile, and aircraft components. Piston scrap contains silicon. Rental points in Moscow accept electrical aluminum with nickel if the component content is no more than 2.8%.
Low grade electrical aluminum - crushed, shavings. The material is sorted by alloy type. A separate class is multi-grade shavings.
Features of supplied aluminum
Electrical aluminum, stripped of insulation, is highly valued at rental points in Moscow. If there are transformers, they must be removed from the bus. The veins are cleaned mechanically, since firing leads to melting and the appearance of streaks on the metal. No paper allowed.
The most valuable aluminum is shiny and clean. The presence of oil is a reason for a decrease in cost due to clogging. If there is a steel core inside, cutting is necessary. Products with traces of oxidation and paint are not accepted. Before delivery, it is necessary to remove the wire grid, damaged parts, and elements containing impurities.
Sources and examples of scrap metal
Electrical aluminum is widely used. These are thin or thick wires and cables used in wiring in homes and large communication networks. In new systems, aluminum parts are less common than durable copper, but in old systems they are widespread. Since many people are replacing wiring with copper, the amount of electrical aluminum that can be recycled is large.
Important and useful
Individuals, enterprises, and large companies can donate electrical aluminum in Moscow
This is not only financially beneficial, but also important for the environment. Recycling and recycling of metal alloys helps protect the environment, maintain the balance of the planet, and protect people from the dangers associated with metal oxidation
Recycled aluminum is used in production processes, which saves costs and conserves resources.
◄ Back to news
Toxicity
It has a slight toxic effect, but many water-soluble inorganic aluminum compounds remain in a dissolved state for a long time and can have a harmful effect on humans and warm-blooded animals through drinking water. The most toxic are chlorides, nitrates, acetates, sulfates, etc. For humans, the following doses of aluminum compounds (mg/kg body weight) have a toxic effect when ingested: aluminum acetate - 0.2-0.4; aluminum hydroxide - 3.7-7.3; aluminum alum - 2.9. Primarily affects the nervous system (accumulates in nervous tissue, leading to severe disorders of the central nervous system). However, the neurotoxicity of aluminum has been studied since the mid-1960s, since the accumulation of the metal in the human body is prevented by its elimination mechanism. Under normal conditions, up to 15 mg of the element per day can be excreted in the urine. Accordingly, the greatest negative effect is observed in people with impaired renal excretory function.
The standard for aluminum content in drinking water is 0.2 mg/l. In this case, this MPC can be increased to 0.5 mg/l by the chief state sanitary doctor for the relevant territory for a specific water supply system.
Conductivity - Aluminum
The electrical conductivity of aluminum depends on the impurity content in it.
The electrical conductivity of aluminum is 62 - 65% of the electrical conductivity of copper. When exposed to air, aluminum is coated with a thin but durable film of oxide, protecting it from corrosion. Mercury salts, alkalis, hydrochloric acid and some of its salts strongly corrode it.
The electrical conductivity of aluminum decreases with the addition of various metals (especially manganese and titanium). Alloys of the hard alloy type have the lowest electrical conductivity in the quenched state and the highest in the annealed state. The cutting machinability of pure aluminum is poor.
| Chemical composition of aluminum. |
The electrical conductivity of aluminum depends on the degree of purity of the metal and decreases with increasing impurity content.
The electrical conductivity of aluminum, used instead of copper for wires, is 65% of the electrical conductivity of copper.
The electrical conductivity of aluminum is highly dependent on impurities and little on mechanical and heat treatment. The purer the aluminum composition, the higher its electrical conductivity and better resistance to chemical influences. Machining, rolling and annealing significantly affect the mechanical strength of aluminum. Cold working increases its hardness, elasticity and tensile strength.
The electrical conductivity of aluminum is 60% of the electrical conductivity of copper, and the density is 3 2 times less than that of copper. Thus, the mass of aluminum wire with equal electrical conductivity is approximately 2 times less. However, the mechanical properties of aluminum, such as strength and fluidity, are significantly lower than those of copper.
The electrical conductivity of aluminum depends on the degree of purity of the metal and decreases with increasing impurity content.
The electrical conductivity of aluminum is 60% of the electrical conductivity of copper.
The electrical conductivity of aluminum is greatly influenced by its purity.
Various impurities affect the electrical conductivity of aluminum, but to varying degrees. The impurities of chromium, vanadium and manganese most strongly reduce electrical conductivity. To a small extent, the electrical conductivity of aluminum depends on the degree of its deformation and the heat treatment regime. The negative effect of deformation on electrical conductivity is eliminated by annealing.
| Microstructure of a wire made of ordinary copper after annealing for 30 minutes in a hydrogen environment at 850 C.| Microstructure of oxygen-free copper wire after annealing for 30 minutes in hydrogen at 850 C. |
Si impurities sharply reduce the electrical conductivity of aluminum, since they form an Al-Si solid solution with aluminum. Iron does not form a solid solution with aluminum, so its effect on the electrical conductivity of the wire is small.
Since vanadium impurities reduce the electrical conductivity of aluminum, contamination of aluminum hydroxide with sodium vanadate is unacceptable. Rinsing the aluminum hydroxide with hot water will usually remove the sodium vanadate fairly completely. With a relatively high V2O5 content in bauxite, special measures must be taken to remove sodium vanadate from the cycle. To do this, part of the circulating solution is cooled to 25 - 30 C. During cooling, vanadium sludge, which is a mixture of soda, phosphate and sodium vanadate, falls out of the solution. Vanadium sludge is a source of vanadium.
The physical properties of aluminum depend on its purity
Basic propertiesAluminum is a chemical element of the third group of the periodic table of D.I. Mendeleev. Table of physical properties of aluminum | |
| Melting point Tmelt, °C | 660 |
| Boiling point Bp, °C | 2 327 |
| Latent heat of fusion, J/g | 393,6 |
| Thermal conductivity l, W/m •deg (at 20° C) | 228 |
| Heat capacity Ср, J/(g deg) (at 0–100°С) | 0,88 |
| Linear expansion coefficient α × 10-6, 1/°С (pro°С) | 24,3 |
| Electrical resistivity ρ × 10-8, Ohm × m (at 20°C) | 2,7 |
| Tensile strength σ in, MPa | 40–60 |
| Relative elongation δ, % | 40–50 |
| Brinell hardness HB | 25 |
| Modulus of normal elasticity E, GPa | 70 |
Aluminum Density
The density of solid and molten aluminum decreases as its purity increases:
Density of aluminum at 20°C
| Purity degree, % | 99,25 | 99,40 | 99,75 | 99.97 | 99,996 | 99.9998 |
| Density at 20°C, g/cm3 | 2,727 | 2,706 | 2,703 | 2,6996 | 2,6989 | 2,69808 |
Density of molten aluminum at 1000°C
| Purity degree, % | 99,25 | 99.40 | 99.75 |
| Density, g/cm3 | 2,311 | 2,291 | 2,289 |
Melting and boiling points.
At the moment of melting of aluminum, the volume of the metal increases: for aluminum with a purity of 99.65% - by 6.25%, for a purer metal - by 6.60%.
As the purity of aluminum increases, its melting temperature increases: Dependence of the melting temperature of aluminum on purity
| Purity degree, % | 99,2 | 99,5 | 99,6 | 99,97 | 99,996 |
| Melting point, °C | 657 | 658 | 659,7 | 659,8 | 660,24 |
Thermal conductivity of aluminum
The thermal conductivity of aluminum increases with increasing degree of its purity. For technical aluminum (99.49 and 99.70%), the thermal conductivity at 200°C is 209 and 222 W/(m×K), respectively. For electrolytically refined aluminum with a purity of 99.9%, the thermal conductivity at 190°C increases to 343 W/(m×K). Impurities of copper, magnesium and manganese in aluminum reduce its thermal conductivity. For example, the addition of 2% Mn to aluminum reduces thermal conductivity from 209 to 126 W/(m×K).
Electrical conductivity of aluminum
Aluminum has high electrical conductivity (fourth place among metals - after silver, copper and gold). The specific electrical conductivity of aluminum with a purity of 99.99% at 20°C is 37.9 μS×m, which is 63.7% of the electrical conductivity of copper [59.5 μS×m]. The purer aluminum [99.999%] has an electrical conductivity equal to 65.9% of copper. The electrical conductivity of aluminum is influenced by a number of factors: the degree of deformation, heat treatment mode, etc., while the nature of the impurities present in aluminum plays a decisive role. Impurities, according to their negative effect on the electrical conductivity of aluminum, can be arranged in the following series: Cr, V, Mn, Ti, Mg, Ag, Cu, Zn, Si, Fe Ni. The impurities of Cr, V, Mn and Ti have the most negative effect on the electrical resistance of aluminum. Therefore, in aluminum for the electrical industry, the sum of Cr+V+Mn+Ti should not exceed 0.015% (grade A5E) and even 0.01% (A7E) with a silicon content of 0.12 and 0.16%, respectively.
The influence of impurities on the electrical conductivity of aluminum
The main impurities in aluminum are silicon, iron, copper, zinc and titanium.
At low silicon contents in aluminum (0.06%), the Fe:Si value (ranging from 0.8 to 3.8) has a relatively small effect on its electrical resistance. As the silicon content increases to 0.15–0.16%, the influence of Fe:Si increases. Below is the effect of Fe:Si on the electrical conductivity of aluminum: Effect of Fe:Si on the electrical conductivity of aluminum
| Fe:Si | 1,07 | 1,44 | 2,00 | 2,68 | 3,56 |
| Electrical resistivity of aluminum, ×10-2 μOhm mm: | |||||
| hard-worked | 2,812 | 2,816 | 2,822 | 2,829 | 2,838 |
| annealed | 2,769 | 2,771 | 2,778 | 2,783 | 2,788 |
The electrical resistivity of annealed aluminum wire (ρ, μΩ m) at 20°C, depending on the impurity content, can be approximately determined by the following formula: ρ=0.0264+0.007×(% Si)+0.0007×(% Fe) + 0.04×[% (Cr+V + Mn + Ti)].
Reflectivity
As the purity of aluminum increases, its ability to reflect light from a surface increases. Thus, the degree of reflection of white light from rolled aluminum sheets (foil), depending on the purity of the metal, increases as follows: for Al 99.2% - 75%, Al 99.5% - 84% and for Al 99.8% - 86 %. The surface of the sheet, made of electrolytically refined aluminum with a purity of 99.996%, reflects 90% of the white light incident on it.
www.metmk.com.ua
Electrical conductivity and current carriers
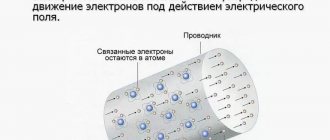
The electrical conductivity of all substances is associated with the presence of current carriers (charge carriers) in them - mobile charged particles (electrons, ions) or quasiparticles (for example, holes in a semiconductor) capable of moving in a given substance over a long distance; in a simplified way, we can say what is meant that such a particle or quasiparticle should be able to travel an arbitrarily large, at least macroscopic, distance in a given substance, although in some particular cases carriers can change, being born and destroyed (generally speaking, sometimes, perhaps, through a very short distance), and carry current, replacing each other.
Since the current density is determined for one type of carrier by the formula:
j→=qnv→cp.,{\displaystyle {\vec {j}}=qn{\vec {v}}_{cp.},} where q{\displaystyle q} is the charge of one carrier, n{\displaystyle n} is the concentration of carriers, v→cp.{\displaystyle {\vec {v}}_{cp.}} is the average speed of their movement,
or j→=∑iqiniv→icp.{\displaystyle {\vec {j}}=\sum _{i}q_{i}n_{i}{\vec {v}}_{icp.}} for more than one type of carrier, numbered by the index i,{\displaystyle i,} taking a value from 1 to the number of types of carriers, each of which can have its own charge (possibly different in magnitude and sign), its own concentration, its own average speed of movement (summation in this formula is implied for all available types of carriers), then, given that the (steady-state) average speed of each type of particle when moving in a specific substance (medium) is proportional to the applied electric field (in the case when the movement is caused precisely by this field, which is what we are here we are considering):
v→cp.=μE→,{\displaystyle {\vec {v}}_{cp.}=\mu {\vec {E}},} where μ{\displaystyle \mu } is a proportionality coefficient called mobility and depending on the type of current carrier in a given specific environment.
It follows that the expression for electrical conductivity is valid:
σ=qnμ,{\displaystyle \sigma =qn\mu ,}
or:
σ=∑iqiniμi{\displaystyle \sigma =\sum _{i}q_{i}n_{i}\mu _{i}} - for more than one type of media.
Conductivity - Aluminum
The conductivity of aluminum, related to the mass of the metal, is 1 5 - 2 times higher than that of copper.
| Dependence of the temperature coefficient of linear expansion (a., specific resistance (p, specific heat capacity (c) of aluminum on temperature. |
Additions of titanium and manganese greatly reduce the conductivity of aluminum. Rolling, drawing and annealing of aluminum are similar to the corresponding operations for copper. Very thin (up to 6 - 7 microns) foil can be rolled from aluminum, used as plates in paper capacitors.
| Examples of the chemical composition of aluminum of various grades. |
SAP is 75% of the conductivity of regular aluminum.
Different impurities reduce the conductivity of aluminum to varying degrees.
All impurities, as in copper, reduce the conductivity of aluminum, which is slightly lower than that of copper.
The cross-section of the wires of line I - 3 is determined by (6 - 19), substituting into it the conductivity of aluminum y32 m / ( Ohm - mm2), the length of network sections, m, active powers flowing through network sections, kW, rated voltage and voltage loss, IN.
| Dependence of magnetic and temperature fields on distance at different moments of dimensionless time. |
Magnetic induction in a conductor with such an abrupt increase in the value of B is shown in Fig. 2 for various moments of dimensionless time; the curves refer to the case where the conductivity corresponds to the conductivity of aluminum.
The results of a study of functional relationships between tolerances on design and technological parameters and the values of nominal energy parameters are presented, for which the number of stator turns, the length of the stator and rotor packages, the conductivity of rotor aluminum, the length of the stator winding turn, the diameter of the wire and the size of the air gap are selected.
| Electrical properties of some pure metals. |
Impurities of arsenic, antimony, cadmium, iron, nickel, cobalt, lead, bismuth, gold, gallium, silicon and zinc at a content of up to 1% slightly reduce the conductivity of aluminum in the annealed state, which is explained by the formation of intermetallic phases. Impurities of copper, silver, and magnesium affect conductivity to a greater extent, while titanium, vanadium, chromium and manganese sharply reduce it, the latter being explained by the formation of solid solutions.
Aluminum wires resist corrosion well, but their mechanical strength and electrical conductivity are lower than those of copper wires. The conductivity of aluminum is 1.65 times less than the conductivity of copper, but aluminum is approximately three times lighter than copper, therefore, with equal copper sections, aluminum wire requires approximately two times less weight than copper. Aluminum easily combines with other metals, which leads to its decomposition, and is very sensitive to mechanical stress, which is why it requires very careful handling.
Aluminum shells have high mechanical strength. Sufficient conductivity of aluminum under certain conditions ensures the use of a three-wire cable sheath as a neutral in a four-wire electrical network, as well as a screen.
Thermal conductivity - aluminum - Great Encyclopedia of Oil and Gas, article, page 3
Thermal Conductivity - Aluminum
Page 3
The temperature of the piston depends on the metal from which it is made. Currently, pistons are usually made of either aluminum or cast iron, with the thermal conductivity of aluminum being three times greater than that of cast iron. Therefore, the heat received by the aluminum piston is quickly removed from the center to its periphery and further into the cylinder walls. [32]
The purity of aluminum is important, since impurities have a significant impact on the electrical, corrosion and technological properties of technical aluminum. In Fig. 457 - 459 shows the effect of impurities and additives on electrical conductivity and
thermal conductivity of aluminum. [34]
The cylinder head is made of gray cast iron or aluminum alloy and is attached to the block on a metal-asbestos gasket with studs or bolts. The GAZ-51 and M-20 Pobeda engines have heads made of aluminum alloy, which makes it possible to slightly increase the compression ratio due to the better thermal conductivity of aluminum and slightly reduces the weight of the engine. [35]
Pure aluminum is used mainly in chemical engineering for the manufacture of equipment and pipelines. Physical properties of aluminum: specific gravity 2 7 g / cm3, melting point 658, boiling point 1800, tensile strength 8 - 10 kgf / mm2, relative elongation 32 - 40%, thermal conductivity of aluminum is three times higher, and the coefficient of linear expansion is two times more than that of iron. [36]
Aluminum and its alloys have high thermal conductivity, heat capacity and latent heat of fusion. The thermal conductivity of aluminum is three times higher than that of low-carbon steel; when heated from 20 to 600 C, the difference in thermal conductivity increases even more. Therefore, welding of aluminum and its alloys must be performed with a relatively powerful and concentrated heat source. [37]
Aluminum and -: h alloys have a high thermal conductivity, 1S1G: pn: mkos1b and latent heat of fusion. The thermal conductivity of aluminum is three times higher than that of low-carbon steel; when heated from 20 to 600 C, the difference in thermal conductivity increases even more. Therefore, welding of aluminum and its alloys must be performed with a relatively powerful and concentrated heat source. [38]
Finally, heat transfer is highly dependent on the wall material. To characterize the heat transfer of various materials, the concept of thermal conductivity is used. Thermal conductivity K is a quantity that shows how much heat is transferred per unit time through a unit wall area: unit thickness with a temperature difference between the wall surfaces equal to one Kelvin. If, for example,
The thermal conductivity of aluminum is 2ShW / (m - K), this means that through each square meter of aluminum wall at a temperature difference of 1 K and with a wall thickness of 1 m, 210 J of heat is transferred within 1 s. [40]
Physical properties are listed in table. 7 - 2 - 1, III. These tables are supplemented by Fig. 7 - 2 - 12 - 7 - 2 - 16A, to which references are made in the tables. The specific gravity of titanium is approximately 1 7 times greater than that of aluminum, and... Its thermal conductivity is relatively low and amounts to /ie the thermal conductivity of aluminum and /6 of iron. [42]
The case of the 2N3375 transistor (case type TO-60) is similar to that discussed in Chapter. TO-63 (see Fig. 4 - 12), but has smaller dimensions. The base of the housing is a copper bolt. A beryllium ceramic plate is soldered onto the upper surface of the bolt head with hard solder. As already noted in § 4 - 1, the unique properties of ceramics based on beryllium oxide lie in the combination of good electrical insulating properties with thermal conductivity almost equal to the thermal conductivity of aluminum. [44]
The difficulty of processing steel molds makes them difficult to manufacture when the mold cavity has a particularly complex configuration. The last method is as follows: molten metal, poured into a preheated mold using the usual casting method, is subjected to pressing on a hydraulic press during the crystallization period. The specific pressure used in this case is 1 - 107 Pa for aluminum alloys. According to another method, the matrices are pressed under a load of 1 4 - 2 1 - 107 Pa into aluminum poured into molds and solidifying. A valuable quality of aluminum is its resistance to the action of sulfur and compounds containing sulfur. The thermal conductivity of aluminum is almost 5 times higher than the thermal conductivity of steel, which leads to a reduction in the vulcanization cycle. Aluminum is resistant to atmospheric influences, and therefore storage of such forms does not require special conditions; Regular dry warehouses are sufficient. [45]
Pages: 1 2 3 4
www.ngpedia.ru
Conductive Adhesive Brands
There are several manufacturers of conductive adhesive, both foreign and domestic, that guarantee high electrical conductivity.
- Contact. Probably the most famous composition among radio amateurs. Conductive adhesive contactol has high elasticity, sufficient strength, is made on a silver basis and dries quickly, which ensures quick and convenient installation. You can buy conductive glue of this brand in any amateur radio store, however, the professionals in this field themselves speak rather poorly about it. But there are also positive reviews. Contactol
- Elekont. Conductive adhesive that will be useful to every car owner. This is an epoxy compound. Reviews about it are also not encouraging. Elekont
- Done deal. This is a foreign representative of this type of glue. Conductive adhesive done deal has increased reliability and strength, which makes it better than domestic analogues. Done Deal
- Homakoll. A fairly popular brand of conductive adhesive that has long established itself in the market. It is used by large companies as an electrically conductive adhesive for floor coverings with an antistatic effect. Homakoll
- Mastix. This company introduces an electrically conductive adhesive for repairing heated rear windows. Mastix conductive adhesive is considered one of the best in this segment. Mastix
- TPK-E. The brand is distinguished by its technical characteristics. This adhesive will function in a wide range of temperatures. From -190 to +200 °C. Used in enterprises.