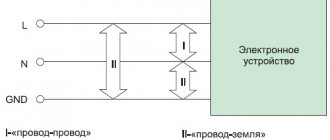
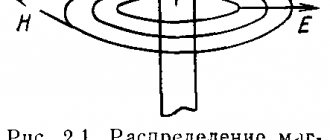
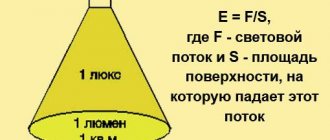
What is electromagnetic radiation?
The term refers to waves that arise when electric and magnetic fields propagate in space are disturbed.
The classification of electromagnetic radiation is based on the frequency spectrum, wavelength and polarization. Polarized EMR refers to where the waves oscillate in one plane. Wavelengths can range from 5 picometers (pm) to tens of kilometers.
Electric charges moving with acceleration generate radiation. Wave propagation occurs both in a dense medium and in a vacuum, but the speed of EMR propagation in matter is lower.
Sources of electromagnetic radiation
The degree of intensity of electromagnetic field radiation is determined by the level of EMR of non-natural origin. High level sources:
- power lines;
- electric transport;
- elevators;
- mobile, television and radio towers;
- transformers.
Low levels of radiation are typical for computer displays, household appliances, and power supply systems. Hard ionizing waves are emitted by medical diagnostic equipment (X-rays, computed tomography). Radiation has wave and particle properties that demonstrate well the phenomenon of the photoelectric effect, where the energy of each electron is determined by the frequency, not the intensity, of the incident light.
An electromagnetic field is produced by moving charges and currents. The theory of the electromagnetic field, created by Maxwell, explains electromagnetic induction: a change in the magnetic field at one point in space leads to the formation of an electric field and vice versa. These fields that generate each other merge into a single electromagnetic field (EMF).
The presence of a closed conductor in the field leads to the appearance of an induction current. At the maximum amplitude of the current and the upward velocity vector of positive charges at all points of the antenna, the charge per unit length is zero.
Electromagnetic spectrum
Before moving on to examples of radiation in physics, it should be noted that each atom emits certain portions of energy. This happens because the states in which an electron can be in an atom are not arbitrary, but strictly defined. Accordingly, the transition between these states is accompanied by the emission of a certain amount of energy.
It is known from atomic physics that photons generated as a result of electronic transitions in an atom have energy that is directly proportional to their oscillation frequency and inversely proportional to the wavelength (a photon is an electromagnetic wave, which is characterized by propagation speed, length and frequency).
Since an atom of a substance can only emit a certain set of energies, this means that the wavelengths of the emitted photons are also specific. The set of all these lengths is called the electromagnetic spectrum.
If the wavelength of a photon lies between 390 nm and 750 nm, then we speak of visible light, since a person can perceive it with his own eyes; if the wavelength is less than 390 nm, then such electromagnetic waves have high energy and are called ultraviolet, x-ray or gamma radiation. For lengths greater than 750 nm, photons have low energy and are called infrared, micro- or radio radiation.
Electromagnetic radiation ranges[ | ]
Electromagnetic radiation is usually divided into frequency ranges (see table). There are no sharp transitions between the ranges; they sometimes overlap, and the boundaries between them are arbitrary. Since the speed of propagation of radiation (in vacuum) is constant, the frequency of its oscillations is strictly related to the wavelength in vacuum.
| Range name | Wavelengths, λ | Frequencies, f | Sources | |
| Radio waves | Extra long | more than 10 | less than 30 k | Atmospheric and magnetospheric phenomena. Radio communication. |
| Long | 10 km – 1 km | 30 kHz - 300 kHz | ||
| Average | 1 km – 100 | 300 kHz - 3 MHz | ||
| Short | 100 m - 10 m | 3 MHz - 30 MHz | ||
| Ultra short | 10 m - 1 mm | 30 MHz - 300 GHz[5] | ||
| Infrared radiation | 1 mm - 780 | 300 GHz - 429 THz | Radiation of molecules and atoms under thermal and electrical influences. | |
| Visible radiation | 780—380 nm | 429 THz - 750 THz | ||
| Ultraviolet | 380nm - 10nm | 7.5⋅1014 Hz – 3⋅1016 Hz | Radiation of atoms under the influence of accelerated electrons. | |
| X-ray | 10 nm - 5 pm | 3⋅1016Hz – 6⋅1019 Hz | Atomic processes under the influence of accelerated charged particles. | |
| Gamma | less than 5 pm | more than 6⋅1019 Hz | Nuclear and space processes, radioactive decay. | |
Ultrashort radio waves are usually divided into meter, decimeter, centimeter, millimeter and decimillimeter waves (hyperhigh frequencies, HHF, 300-3000 GHz) - standard radio wave ranges according to the generally accepted classification [5]. According to another classification, the specified standard ranges of radio waves, excluding meter waves, are called microwaves
or ultra-high frequency waves (microwave)[6].
Ionizing electromagnetic radiation. This group traditionally includes x-rays and gamma radiation, although, strictly speaking, ultraviolet radiation and even visible light can ionize atoms. The boundaries of the regions of X-ray and gamma radiation can be determined only very conditionally. For general guidance, we can assume that the energy of X-ray quanta lies in the range of 20 eV - 0.1 MeV, and the energy of gamma quanta is more than 0.1 MeV. In a narrow sense, gamma radiation is emitted by the nucleus, and X-ray radiation is emitted by the atomic electron shell when an electron is knocked out of low-lying orbits, although this classification is not applicable to hard radiation generated without the participation of atoms and nuclei (for example, synchrotron or bremsstrahlung radiation).
Radio waves[ | ]
Main article: Radio emissions
Due to the large values of λ, the propagation of radio waves can be considered without taking into account the atomistic structure of the medium. The only exceptions are the shortest radio waves adjacent to the infrared part of the spectrum. In the radio range, the quantum properties of radiation have little effect, although they still have to be taken into account, in particular, when describing quantum generators and amplifiers in the centimeter and millimeter ranges, as well as molecular frequency and time standards, when cooling equipment to temperatures of several kelvins.
Radio waves arise when alternating current of the appropriate frequency flows through conductors. And vice versa, an electromagnetic wave passing through space excites a corresponding alternating current in a conductor. This property is used in radio engineering when designing antennas.
The natural source of waves in this range are thunderstorms. It is believed that they are also the source of Schumann's standing electromagnetic waves.
Microwave radiation[ | ]
Main article: Microwave radiation
| This section of the article has not been written. According to the plan of one or more Wikipedia contributors, a special section should be located in this place. You can help by writing this section. This mark was set on February 28, 2021 . |
Infrared radiation (thermal)[ | ]
Main articles: Infrared radiation
and
Thermal Radiation
Like radio and microwaves, infrared radiation (IR) is reflected from metals (as well as from most electromagnetic interference, which is in the ultraviolet range). However, unlike low-frequency radio and microwave radiation, infrared EMR typically interacts with dipoles present in individual molecules, which change when the atoms at the ends of the same chemical bond vibrate.
Consequently, it is absorbed by a wide range of substances, causing their temperature to rise while dissipating vibrations as heat. The same process, occurring in reverse, causes spontaneous emission of massive substances in the infrared (see Thermal Radiation section below).
Infrared radiation is divided into spectral subregions.
Although different division schemes exist, the spectrum is generally divided into near-infrared (0.75–1.4 µm), short-wave infrared (1.4–3 µm), mid-wave infrared (3–8 µm), long-wave infrared (8–15 µm ) and far infrared (15–1000 µm).
| This section of the article has not been written. According to the plan of one or more Wikipedia contributors, a special section should be located in this place. You can help by writing this section. This mark was set on February 28, 2021 . |
Visible radiation (optical)[ | ]
Main article: Visible radiation
A transparent prism decomposes a white beam into its constituent rays[7]
Visible, infrared and ultraviolet radiation make up the so-called optical region of the spectrum
in the broad sense of the word. The identification of such a region is due not only to the proximity of the corresponding parts of the spectrum, but also to the similarity of the instruments used for its study and developed historically mainly in the study of visible light (lenses and mirrors for focusing radiation, prisms, diffraction gratings, interference devices for studying the spectral composition of radiation and etc.).
The frequencies of waves in the optical region of the spectrum are already comparable to the natural frequencies of atoms and molecules, and their lengths are comparable to molecular sizes and intermolecular distances. Thanks to this, phenomena caused by the atomic structure of matter become significant in this area. For the same reason, along with the wave properties, the quantum properties of light also appear.
The most famous source of optical radiation is the Sun. Its surface (photosphere) is heated to a temperature of 6000 K and shines with bright white light (the maximum of the continuous spectrum of solar radiation is located in the “green” region of 550 nm, where the maximum sensitivity of the eye is located). It is precisely because we were born near such a star that this part of the spectrum of electromagnetic radiation is directly perceived by our senses.
Radiation in the optical range occurs, in particular, when bodies are heated (infrared radiation is also called thermal radiation) due to the thermal movement of atoms and molecules. The more a body is heated, the higher the frequency at which the maximum of its radiation spectrum is located (see: Wien's displacement law). When heated to a certain level, the body begins to glow in the visible range (incandescence), first red, then yellow, and so on. And vice versa, radiation from the optical spectrum has a thermal effect on bodies (see: Bolometry).
Optical radiation can be created and detected in chemical and biological reactions. One of the most famous chemical reactions, which is a receiver of optical radiation, is used in photography. The source of energy for most living beings on Earth is photosynthesis, a biological reaction that occurs in plants under the influence of optical radiation from the Sun.
Ultraviolet radiation[ | ]
Main article: Ultraviolet radiation
| This section of the article has not been written. According to the plan of one or more Wikipedia contributors, a special section should be located in this place. You can help by writing this section. This mark was set on February 28, 2021 . |
Hard radiation[ | ]
Main articles: X-rays
and
Gamma Radiation
In the field of X-rays and gamma radiation, the quantum properties of radiation come to the fore.
X-ray radiation occurs when fast charged particles (electrons, protons, etc.) decelerate, as well as as a result of processes occurring inside the electronic shells of atoms. Gamma radiation appears as a result of processes occurring inside atomic nuclei, as well as as a result of the transformation of elementary particles.
Types of electromagnetic radiation
EMR is divided into types according to length and frequency characteristics.
The wavelength varies in the following ranges:
Electromagnetic radiation ranges
- Radio waves (from 0.1 mm to 10 km or more) are divided into short, ultra-short, medium, long and ultra-long. Ultrashort radio waves belong to ultra-high frequency (microwave) waves.
- Infrared rays (from 1 mm to 780 nm).
- Ultraviolet rays (from 380 mm to 10 nm).
- Visible light (from 780 mm to 380 nm).
- X-ray radiation (from 10 nm to 5 pm).
- Gamma rays (up to 5 pm).
The frequency of the waves varies from 30 kHz (for radio waves) to 6×10¹9 Hz or more (for gamma rays).
Waves of different lengths are formed in different ways:
- X-rays appear when rapidly moving electrons pass into a state with lower energy due to braking;
- ultraviolet is emitted due to the movement of accelerated electrons;
- infrared radiation is emitted by hot objects;
- radio waves are formed from high-frequency currents moving through antennas;
- Ionizing gamma radiation is emitted during nuclear reactions.
The above types of waves are absorbed differently by substances: X-ray and gamma waves penetrate the tissues of the body and are almost not absorbed, infrared rays pass through a number of opaque objects, and when absorbed, the substance heats up.
Properties of electromagnetic waves
The most important result that follows from the theory of the electromagnetic field formulated by Maxwell was the prediction of the possibility of the existence of electromagnetic waves. Electromagnetic wave
– propagation of electromagnetic fields in space and time.
The source of the electromagnetic field is electric charges moving with acceleration.
Electromagnetic waves, unlike elastic (sound) waves, can propagate in a vacuum or any other substance.
Electromagnetic waves in vacuum propagate at a speed of c = 299,792 km/s
, that is, at the speed of light.
In matter, the speed of an electromagnetic wave is less than in a vacuum. The relationship between the wavelength, its speed, period and frequency of oscillations obtained for mechanical waves is also true for electromagnetic waves:
Fluctuations of the tension vector E
and the magnetic induction vector
B
occur in mutually perpendicular planes and perpendicular to the direction of wave propagation (velocity vector).
An electromagnetic wave transfers energy.
Electromagnetic wave range
Around us is a complex world of electromagnetic waves of various frequencies: radiation from computer monitors, cell phones, microwave ovens, televisions, etc. Currently, all electromagnetic waves are divided by wavelength into six main ranges.
Radio waves
– these are electromagnetic waves (with a wavelength from 10,000 m to 0.005 m), used to transmit signals (information) over a distance without wires. In radio communications, radio waves are created by high-frequency currents flowing in an antenna.
Electromagnetic radiation with a wavelength from 0.005 m to 1 micron, i.e. lying between the radio wave range and the visible light range are called infrared radiation
. Infrared radiation is emitted by any heated body. The sources of infrared radiation are stoves, batteries, and incandescent electric lamps. Using special devices, infrared radiation can be converted into visible light and images of heated objects can be obtained in complete darkness.
Towards visible light
include radiation with a wavelength of approximately 770 nm to 380 nm, from red to violet. The significance of this part of the spectrum of electromagnetic radiation in human life is extremely great, since a person receives almost all information about the world around him through vision.
Electromagnetic radiation with a wavelength shorter than violet, invisible to the eye, is called ultraviolet radiation.
It can kill pathogenic bacteria.
X-ray radiation
invisible to the eye. It passes without significant absorption through significant layers of a substance that is opaque to visible light, which is used to diagnose diseases of internal organs.
Gamma radiation
called electromagnetic radiation emitted by excited nuclei and arising from the interaction of elementary particles.
Electromagnetic wave scale
According to modern concepts, light is a stream of electromagnetic field particles called photons and having dual corpuscular-wave properties (i.e. light has the properties of a stream of particles and waves). The main characteristic of light waves is the oscillation frequency ν (the frequency of oscillations of the strength vectors E and H of the electromagnetic field). More often, the associated wavelength in vacuum is used: λ = cT = c/ν, where c is the speed of light in vacuum, rounded off as c = 3•108 m/s, T is the oscillation period.
In accordance with the excitation conditions and radiation properties, electromagnetic waves are divided by frequency (or wavelength) into several ranges that make up the electromagnetic wave scale: radio waves, optical radiation, x-rays, gamma radiation. The boundaries of these ranges are arbitrary, since they are largely determined by radiation sources and therefore can overlap (Fig. 1).
Fig.1
| Fig.2. |
Electromagnetic radiation with wavelengths ranging from 400 microns to 10 nm is called optical radiation. Optical radiation within the wavelength range from 760 to 380 nm, acting on the eye, causes the sensation of light. It is called visible radiation. In the direction of longer waves from it in the spectrum there is invisible infrared radiation, in the direction of shorter waves - invisible ultraviolet.
The radiation can be simple (or monochromatic) and complex. Radiation of any one wavelength is called monochromatic. This is an idealized view; Radiation in which the wavelengths of its constituent waves differ by no more than tenths of a nanometer is considered practically monochromatic. Monochromatic radiation in the visible part of the spectrum of a certain wavelength, acting on the eye, causes the sensation of the corresponding color. Radiation consisting of waves of different lengths is called complex. Depending on its spectral composition, it can cause different color sensations,
| Fig.3 |
Among the many possible types of complex radiation, white light is distinguished. White light refers to the visible part of the radiation from the Sun (Fig. 2), as well as radiation from opaque solids and liquids heated to a high temperature (several thousand degrees). This radiation contains all waves of the visible range in a certain intensity ratio.
Range.
The spectrum of electromagnetic radiation is a set of monochromatic waves, ordered by length, into which light or other electromagnetic radiation is decomposed. A typical example of a spectrum is the well-known rainbow. The possibility of decomposing sunlight into a continuous sequence of rays of different colors was first experimentally demonstrated by I. Newton in 1666. By directing a narrow beam of light onto a triangular prism (Fig. 3), which penetrated into a darkened room through a small hole in the window shutter, he received an image of a painted wall on the opposite wall. stripes with a rainbow alternation of colors, which he named with the Latin word spectrum. Conducting experiments with prisms, Newton came to the following important conclusions: 1) ordinary “white” light is a mixture of rays, each of which has its own color; 2) rays of different colors, refracted in a prism, are deflected at different angles, as a result of which “white” light is decomposed into colored components.
Study of spectra. Spectral devices.
The dispersion of complex radiation in a triangular prism of a transparent substance (for visible light - heavy flint glass, for ultraviolet radiation - quartz and for infrared - rock salt or sylvite) is used to design devices for studying the spectrum and measuring the wavelength of complex radiation (spectroscopes and spectrographs ).
| Fig.4 Prism spectroscope. a - optical design and ray path, b - appearance and components of the device |
The simplest prism spectroscope (Fig. 4, b) consists of a tripod O, on which a horizontal disk D with divisions is mounted. A prism P is installed in the center of the disk; two pipes are located at the edges of the disk: collimator K and visual 3, which can be installed at the required angle using screw B. The collimator (Fig. 4, a) has a slit at the end, in front of which a light source is placed; lens O forms a beam of parallel rays, which is necessary so that the rays passing through the prism also consist of parallel beams. These beams are focused by the telescope lens O2 into its focal plane FF and form each slit image of the corresponding color, which is called a spectral line. The combination of these lines forms the spectrum under study, which is observed in an enlarged form through the eyepiece Ok. A spectrograph (Fig. 5: a - general view and b - diagram of the device) is a more complex device, adapted for photographing spectra. Light through the slit D and lens L1 is directed to the dispersion prism P, beams of spectrally decomposed light are focused by lens L2 on the photographic plate F.
| Rice. 5 |
Using a spectral device, you can obtain monochromatic light of the required wavelength. To do this, a slit diaphragm is placed in the focal plane of the second lens L2, with the help of which the desired line is isolated from the spectrum. Such a device is called a monochromator.
Classification of spectra.
All spectra are divided into two main classes: emission (or emission) spectra and absorption spectra. Each class, in turn, is divided into continuous (solid), striped and line spectra.
Spectra consisting of bright lines or stripes on a dark background are called emission spectra. They occur when a substance is extremely hot or bombarded with electrons. Absorption spectra, consisting of dark areas against a bright background, are produced when white light passes through a translucent medium that absorbs certain frequencies.
Fig.6. Emission spectrum of iron Fe
| Fig.7 Examples of optical spectra. Emission spectra: 1-solar, 2-sodium, 3-hydrogen, 4-helium Absorption spectra: 5-solar, 6-sodium, 7-hydrogen, 8-helium |
A line spectrum is a spectrum in which only certain wavelengths, or “lines,” appear. The banded spectrum consists of lines grouped into bands. Emission and absorption spectra are individual for each substance, so they are used to identify substances in the science of spectroscopy. Spectra are the result of electron transitions between different energy levels in atoms or molecules of a substance, resulting in the emission or absorption of electromagnetic radiation.
Emission spectra (emission spectra) are excited for vapors and gases by electrical discharge, and for liquids and solids by heating to a high temperature, for example in a colorless flame of a gas burner. For organic substances that are degraded by high temperatures, absorption or absorption spectra are usually studied. The absorption spectrum is the set of dark lines or stripes formed in the continuous spectrum of white light when it passes through a given transparent medium. To obtain an absorption spectrum in a spectral device, a test substance, for example a plane-parallel cuvette with a test solution, is thrown between a source of white light (for example, an electric arc and a collimator slit or between a collimator and a prism).
Bopa's theory. Spectrum of a hydrogen atom
In 1913, N. Bohr proposed a theory of the mechanism of light emission by atoms, taking into account the quantum nature of light. The theory is based on two postulates:
1. The internal energy of an atom is discrete; it can only take on certain permitted values (or levels) characteristic of a given atom. The states of the atom corresponding to these energy levels are stationary: in this state, the atom does not emit electromagnetic waves, despite the movement of electrons occurring in it.
2. When an atom transitions from one stationary state to another, monochromatic electromagnetic radiation is emitted (or absorbed), the frequency of which is determined by the energy equal to the difference between the energy levels E2 and E1 corresponding to these states:
where h is Planck's constant.
| Table 1. Energy values at various levels in the hydrogen atom | |
| Principal quantum number | Energy level |
| n=1 | E1 = -13.55 eV(ground) |
| n=2 | E2 = -3.88 eV |
| n=3 | E3 = -1.5 eV |
| n=4 | E4 = -0.84 eV |
| n=5 | E5 = -0.54 eV |
| n=6 | E6 = -0.38 eV |
Using the nuclear model of the atom, Bohr proposed to assume that the stationary states, or allowed energy levels, of the atom correspond to the movement of electrons in orbits of a certain radius.
Based on the quantization condition, Bohr calculated the energy levels for the hydrogen atom. In an atom, an electron is held in orbit by the force of Coulomb attraction to the nucleus, which causes centripetal acceleration.
For the first, main orbit, the radius is r1 = 0.53•10-8 cm, which is consistent with calculations based on the kinetic theory of gases. The speed of electron motion in a stationary orbit for the main orbit of the hydrogen atom is v1= 2.3•108 cm/s. This is the order of the speed of electron motion in orbit.
| Fig.8 Electron transitions in a hydrogen atom: a – excitation and emission of 1 quantum with energy ΔE=E3 - E1 b – excitation and emission of 2 quanta with energies E3-E2 and E2-E1 |
The total energy of the electron is Ev = Ek + En, and the potential energy depends on the radius of the orbit. Energy levels are inversely proportional to the square of the quantum number and their values are presented in Table 1.
Since the negative values of the electron energy decrease in absolute value with increasing orbital radius, we can assume that the energy levels increase.
Thus, as the distance from the nucleus increases, the energy levels of the atom increase:
E1 < E2 < …
As the number n increases, the difference between each two adjacent levels decreases in absolute value:
ΔE'> ΔE'' > ΔE''' > …
where ΔE'=E2-E1; ΔE''=E3–E2; ΔE'''=E3–E2. The stationary level with the lowest energy is called the ground level; it corresponds to the state of the atom, which is not subject to any external influences. The remaining stationary levels are called excited. Excitation of an atom, i.e., the transition of an electron to an orbit of a larger radius, requires the communication of additional energy and, therefore, occurs as a result of any external influences: the collision of particles in the process of intense thermal motion, electrical discharge in gases, absorption of electromagnetic radiation, etc. as a result of the recombination of ions in a gas or electrons and holes in a semiconductor, when an atom is exposed to radioactive radiation and some other influences.
The excited state of the atom is unstable; after about 10-8 s, the electron returns to the main orbit, while one photon with energy hv is emitted, equal to the energy obtained during excitation (Fig. 8, a), and the atom goes into the ground state. An electron can return to the main orbit not only through a single transition, but also through intermediate levels. In this case, during the transition, several photons with energies hv' and hv" will be emitted, equal to the difference in the energies of these levels (Fig. 8, b).
Bohr's theory explained not only the origin of line spectra, but also the structure of the emission spectrum of hydrogen atoms. Depending on the energy obtained when the atom is excited, the electron goes to different excited levels. When it returns to the main level (especially if this transition occurs stepwise), quanta of various energies are emitted. Therefore, in the emission spectrum of a hydrogen atom there must be a significant number of lines, the arrangement of which corresponds to the energy levels of the atom and possible electron transitions.
Even before the creation of Bohr's theory, it was established that in the spectrum of hydrogen there are groups, or series, of lines, the frequencies of which are in certain proportions among themselves, for example, the Lyman series (in the ultraviolet part of the spectrum), Balmer (in the visible part of the spectrum), Paschenne ( in the infrared region), etc.
Fig.9. Spectral series of the hydrogen atom. a - electron transition, b - energy levels, c - location of lines in the radiation spectrum (visible region of the spectrum, see Fig. 7).
Bohr's theory explained the origin of these series (Fig. 9). The energy of emitted photons is equal to the difference between the energy levels En and Em of the electron transition: hv = En - En0, whence v = (En - En0)/h. Thus, according to Bohr's theory, the Lyman series, for example, includes all transitions of electrons from excited levels (n = 2, 3, 4, ...) to the ground level (n0 = 1), the Balmer series includes transitions from higher levels (n - 3, 4, 5, ...) to the first excited level (n0 = 2), etc.
Bohr's theory was confirmed in the spectral patterns of the hydrogen atom. However, an attempt to apply it to the spectra of more complex atoms encountered significant difficulties.
Molecular spectra
If the energy imparted to the atom is insignificant, then mainly valence electrons move to excited levels. The radiation frequency corresponds to the optical part of the spectrum (visible and parts of infrared and ultraviolet radiation close to it). For atoms with a high atomic number, a higher excitation energy causes electron transitions between levels corresponding to the inner layers. Radiation during electron transitions between these levels has a significantly higher frequency and belongs to the far ultraviolet and x-rays.
Molecules have more complex emission (or absorption) spectra than atoms of the same substance. When atoms are combined into a molecule, the configuration of the shell with valence electrons changes, energy bands are formed in solids, and therefore the number of possible electron transitions and the corresponding spectral lines increases significantly.
In addition to the levels (Ee) associated with electron transitions, molecules have energy levels (Em), caused, firstly, by the vibrational motion of the atomic nuclei that form the molecule near the equilibrium position (Ecol), and, secondly, by the rotational motion of the molecule itself (Eur). The energy of these types of motion is also quantized, that is, it has its own allowed (quantized) energy levels. Thus, molecular spectra consist of three components - electronic, vibrational and rotational. External influences enhance the intensity of these types of molecular motion, i.e., they excite the molecule, which then returns to the ground state, emitting a photon with an energy equal to the difference in the energy levels of the transition.
All these components produce in the spectrum many closely spaced lines, which together form striped (mainly for vapors and gases) or continuous (for solids and liquids) spectra.
As theory and experience show, Eur <col <<Ee, therefore molecular spectra occupy wide ranges of electromagnetic radiation, with the rotational and vibrational components belonging primarily to infrared radiation, and the electronic components to visible and ultraviolet radiation.
Analysis of molecular spectra, especially in the infrared region, is widely used in studying the structure of molecules.
The rotational component of molecular spectra can also occupy the region of short radio waves. The study of emission and absorption spectra in this range is called radio spectroscopy. These data complement the information on the structure of molecules obtained using optical spectroscopy.
The most common radiospectroscopy method is electron paramagnetic resonance (EPR). In the case of non-paramagnetic bodies, the phenomenon of nuclear magnetic resonance (NMR) is used for similar purposes.
The excited state of an atom or molecule can be resolved not only by emitting a photon. It can cause a photochemical reaction, a restructuring of the structure of a complex molecule, and the energy obtained during excitation can be transferred to other particles in the process of thermal motion. These phenomena are called non-radiative energy transitions.
Scattering and absorption of light. Bouguer-Lambert-Beer law.
When passing through a material medium, a light wave gradually weakens. This occurs due to the scattering and absorption of light.
Light scattering occurs in inhomogeneous media, provided that the size of the inhomogeneities is commensurate with the wavelength of light. If the heterogeneity of the medium is formed by foreign particles randomly distributed in the mass of the medium, then light scattering is called the Tyndall phenomenon, and the medium is turbid, for example, fine fog, smoke, various suspensions and emulsions, etc. This phenomenon can be observed, for example, when a narrow beam Sun rays pass through a dusty atmosphere: the light is scattered on dust particles and the entire beam becomes visible when observed from any direction.
The wavelength of light does not change during scattering, and the intensity of the scattered light is higher, the smaller the size of these inhomogeneities compared to the wavelength. The intensity of scattering also depends on the wavelength of light: short waves are scattered much more strongly than long ones. We can assume that the intensity of the scattered light is inversely proportional to approximately the second power of the wavelength for larger particles and the third power for smaller particles. Therefore, for example, fine fog is blue, and that consisting of larger droplets is white.
Light scattering can also occur in a homogeneous medium on instantaneous inhomogeneities (fluctuations) in the density of matter formed in connection with the thermal movement of atoms and molecules, for example, in a pure gas, during thermal motion, molecules at various moments come closer to one another in some points of the gas volume and become rarefied at others . This type of scattering is called molecular scattering. The intensity of the scattered light is inversely proportional to the fourth power of the wavelength of the incident light (Rayleigh's law). In this regard, for example, the glow of the sky is observed to be blue-blue, and direct solar radiation acquires a yellow-red hue, especially at sunrise and sunset, when this radiation travels a longer path in the atmosphere.
When light is scattered in homogeneous liquids and crystals, in scattered light, in addition to the incident wave with frequency ω0, waves with frequency ωm appear, differing from it by a certain value Δω, characteristic of the molecular structure of a given substance. This type of molecular scattering is called Raman scattering and is important for studying the structure of matter.
| Rice. 10 |
When light is scattered, energy retains its electromagnetic nature. When light is absorbed, it transforms into other types of internal energy, and various phenomena can occur in the substance: an increase in the intensity of thermal motion (thermal effect), excitation and ionization of atoms and molecules, activation of molecules (photochemical effect), etc.
The law of absorption in a homogeneous medium for a parallel beam of monochromatic light was established by N. Bouguer: in each subsequent layer of a medium of the same thickness, the same part of the energy flux of the light wave incident on it is absorbed, regardless of its absolute value.
Based on this law, let us determine the intensity Id of a light wave passing through a layer of a medium of thickness d if the wave incident on the surface of the medium has intensity I0. To do this, we select a layer of the medium with a thickness dx at a distance x from the surface (Fig. 10, a). The decrease dIx in the intensity of the Ix wave due to the absorption of light by this layer according to Bouguer’s law is proportional to the value of Ix and the thickness of the layer dx:
where α is the proportionality coefficient. The equation can be given the form dIx/Ix = - α dx.
Solving this equation, we obtain for a layer of thickness x = d
A graph of changes in light intensity Ix depending on the thickness of the layer of the medium through which the light passes is shown in Fig. 10, b (exponential curve).
The proportionality coefficient α is called the absorption index and characterizes the absorption capacity of a substance.
It depends on its nature and state, as well as on the frequency (wavelength λ0) of light. For metals, the absorption rate is very high (about 103-108 cm-1). This is explained by the presence of free electrons in metals, the forced vibrations of which are easily excited and have a significant amplitude. A light wave incident on the surface of a metal quickly consumes its energy and therefore penetrates to the smallest depth.
In dielectrics, the absorption index is generally low (about 10-3 – 10-5 cm-1), but they exhibit selective absorption of light in certain wavelength ranges, in which the absorption index increases sharply. This is due to the fact that there are no free electrons in dielectrics and significant absorption of light occurs only during resonant oscillations, i.e., at light wave frequencies close to the natural (or multiples of them) oscillation frequencies of the dielectric electrons. This phenomenon explains, for example, the line absorption spectra of gases in the atomic state.
| Fig.11 |
The approximate nature of the dependence of the absorption coefficient on wavelength k is shown in Fig. 11. In Fig. 11, a - graph 1 for bodies that uniformly absorb light of any wavelength (black and gray bodies), 2 - for bodies that absorb light of any wavelength starting from a certain boundary λgr, 3 - for bodies that have a wide absorption band within the range of wavelengths waves from λ1 to λ2. In Fig. 11 b - for bodies with selective (resonant) absorption at certain wavelengths λ1, λ2 and λ3.
The gradual decrease in light intensity when passing through a medium due to scattering also obeys Bouguer’s law, the formula of which, taking into account both absorption and scattering, takes the form
| Rice. 12. Attenuation of light from intensity I0 to Id when passing through a layer of substance (solution) of thickness d, with concentration c and absorption (extinction) coefficient α. |
where σ is the indicator of light attenuation due to scattering.
Studying the absorption of monochromatic light by solutions of colored substances (provided that the solvent does not absorb light of a given wavelength and the solution has a low concentration), A. Beer showed that it obeys Bouguer’s law, and the absorption coefficient α is directly proportional to the concentration of the substance in the solution (Beer’s law ): α = χС, where χ is the absorption index for a solution of unit concentration. Then the formula of the Bouguer-Lambert-Beer law will take the form
or in the system of decimal logarithms, where
The ratio Id/I0 = τ is called the transmittance or transparency of the solution, and the value D = log (Id/I0) = -log τ is the optical density. In accordance with the above formula, the optical density of the solution is D = χ'Cd.
| Fig.13 |
The Bouguer-Beer law is based on a method for determining the concentration of solutions by comparing the thicknesses d1 and d2 of layers of two solutions of the same substance, studied with a concentration of C1 and standard C2, in which the same absorption of light occurs. In an instrument called a concentration colorimeter, light from the same source passes through layers d1 and d2 of solutions; by changing the thickness of the layers, the brightness of the two halves of the field of view illuminated by the light passing through these solutions is equalized (Fig. 13). In this case, the optical densities of the solutions are also equalized: D1 = D2, or C1d1 = C2d2, whence C1/C2 = d1/d2, i.e., the concentrations of C1 and C2 are inversely proportional to the thicknesses of the layers d1 and d2.
A similar method for determining the concentration of a substance in a colloidal solution is called nephelometry. In this case, the intensities of light scattered by particles in the standard and test solutions are compared: at relatively low concentrations they are proportional to the concentration of suspended particles and the height of the solution column. The solutions are illuminated by side light.
Spectral analysis
Spectra (both emission and absorption) are closely related to the structure of atoms and molecules of a substance. Therefore, by their character one can judge the nature and composition of both simple and complex substances. The method of qualitative or quantitative determination of the composition of a substance by its spectrum is called spectral analysis. Its main advantage is that an extremely small amount of the substance is required for analysis. By means of spectral analysis, the presence of a substance in quantities of up to 10-8 g can be detected. Using spectral analysis, for example, it was found that living organisms contain many metals in extremely small quantities - cobalt, chromium, titanium, etc. Spectral analysis makes it possible to identify traces of blood (forensic medicine), trace metals in canned products (food hygiene, etc.).
Absorption spectrophotometry.
Absorption spectroscopy is used to study the molecular composition of organic substances; usually, the substance under study is dissolved in water, which itself does not provide an absorption spectrum in the visible light region. Using absorption spectroscopy, for example, the molecular composition of many vitamins, hormones, etc. was established.
| Fig. 14. Absorption spectra of hemoglobin and its compounds in visible light: 1 - hemoglobin; 2 - oxyhemoglobin; 3 - carboxyhemoglobin; 4 - methemoglobin: |
The use of visible and ultraviolet absorption spectrophotometry for quantitation techniques is based on the fact that the absorbance of a substance is usually a constant independent of the intensity of the incident radiation, the length of the cuvette and the concentration, so that the concentration can be determined photometrically. Deviations from the above values may be due to physical, chemical or instrumental variables. Deviations due to instrumental error can be caused by the influence of slit width, light scattering or polychromatic radiation. Apparent errors may also result from changes in the concentration of solute molecules due to association between solute molecules, between solute and solvent molecules, or due to dissociation or ionization
The property of atoms and molecules to absorb light with a certain wavelength characteristic of a given substance is widely used in medicine and pharmacy for qualitative and quantitative research. Measuring absorption spectra allows one to judge the chemical composition of a substance and its state in biological structures. Spectrophotometers are used to record absorption spectra.
Absorption spectrum - often expressed graphically, the ratio of absorption or any absorption function to wavelength or any function of wavelength (see Fig. 7, 11). The absorption spectra of substances are determined by the energy difference between the energy levels of the molecules that make up the substance, as well as the probabilities of transition between them. The energy difference determines the wavelength at which light is absorbed, and the transition probability determines the absorption coefficient of the substance. Biologically important molecules are characterized by broad absorption bands due to electronic, vibrational and rotational levels. Molecular groups that absorb light are called chromophores.
Standard measurement range in absorption spectrophotometry: 180-1100 nm. It includes three spectral regions: near ultraviolet region (UV) -180-380 nm; visible (VIS) - 380-760 nm and near infrared (IR) - 760-1100 nm.
Nucleic acids absorb only in the UV region (180-220 and 240-280 nm). Their chromophores are mainly purine and pyrimidine bases.
Proteins have three types of chromophoric groups: the peptide groups themselves, side groups of amino acid residues and prosthetic groups. The first two absorb in the UV region and do not absorb in the visible region. Peptide groups -CO-NH- absorb around 190 nm. The side groups of three aromatic acids—tryptophan, tyrosine, and phenylalanine—also absorb at these wavelengths, much more strongly than the peptide groups. In addition, they have an absorption band in the range of 260-280 nm.
Prosthetic groups (heme in hemoglobin and other chromophores) absorb in the UV and visible region. They are what gives protein its color (for example, red color to hemoglobin). The absorption spectrum of hemoglobin (Fig. 15) has characteristic maxima in the visible region (~400 nm and 525-580 nm), as well as in the near-IR region (900 nm). The absorption spectra of hemoglobin that has bound oxygen (oxyhemoglobin) - red line and free hemoglobin (deoxyhemoglobin) - blue line are different. Therefore, using absorption spectra, it is possible to measure the oxygen content in human blood.
Fig. 15. Absorption spectra of hemoglobin and oxyhemoglobin in the optical radiation region
Examples of the use of spectrophotometry in biology, medicine and pharmacy.
· Measurement of protein and nucleic acid concentrations.
· Assessment of blood supply to tissues based on measurements of the degree of oxygenation of hemoglobin.
· Measuring the pH of the environment using dyes that change the absorption spectrum with changes in pH.
· Determination of the concentration of various drugs with characteristic absorption spectra (rutin, berberine).
· Monitoring the dynamics of the reproduction of microorganisms by changes in the optical density of the environment in which they are located.
Schematic diagram of a spectrophotometer.
The spectrophotometer consists of the following main blocks (Fig. 16): light source (I), monochromator (M), measuring cell (K1) and comparison cell (K2), photodetector (F) and recorder (indicator) (P).
| Fig. 16. Schematic diagram of a spectrophotometer |
The source (I) emits light, the monochromator (M) selects the desired part of the spectrum from it. This light then passes either through the measuring cuvette (K1), into which the test solution is placed, or through the reference cuvette (K2), filled with a solvent (in this case, cuvette K2 is placed instead of cuvette K1). The light passing through the cuvette is recorded by a photodetector (F), and its intensity is either recorded by some kind of recorder or displayed on an indicator. A pointer device can be used as an indicator. Two cuvettes are used to eliminate parasitic effects associated with the absorption of light in the solvent and its reflections from the walls of the cuvette.
Radiation sources
According to the nature of their occurrence, EMR sources can be artificial (electrical devices and mechanisms) and natural (Earth's field, atmospheric phenomena, nuclear fusion).
Radiation moves from source to receiver at high speed. According to most theories, if they are separated by vacuum space or rarefied gas, the speed of movement of the waves is equal to the speed of light (300 thousand km/s).
All types of radiation move equally quickly in free space, only the frequency of oscillations per second will be different.
Sources of electromagnetic radiation in everyday life
Sources of electromagnetic radiation:
- heated bodies (incandescent lamps, radiators);
- radioactive elements;
- power lines;
- radio and television transmitters;
- laser installations;
- cellular communication stations;
- radar and relay stations;
- nuclear and space processes;
- railway and electric transport;
- household electrical appliances.
There are sources of electromagnetic waves in every apartment (TVs, refrigerators, microwave ovens, Wi-Fi routers, mobile phones). Air fryers, defrost refrigerators, microwave ovens, cell phones and computers pose the greatest electromagnetic hazard. The closer a person is to the source and the higher its power, the greater the impact EMR has on the body.
Power lines
The wires of a working power transmission line (PTL) create electromagnetic fields of industrial frequency in the adjacent space.
The distance over which these fields extend from the line wires reaches tens of meters. The range, propagation and magnitude of the field depend on the voltage class of the power line (the number indicating the voltage class is in the name - for example, a 220 kV power line), the higher the voltage, the larger the zone of increased electromagnetic field level, while the size of the zone does not change during operation power lines.
Since the load on power lines can change repeatedly both during the day and with changing seasons, the size of the zone of increased magnetic field level also changes. The boundaries of sanitary protection zones for power lines on existing lines are determined by the criterion of electric field strength - 1 kV/m. The placement of ultra-high voltage overhead lines (750 and 1150 kV) is subject to additional requirements regarding the conditions of exposure to the electric field on the population. Thus, the closest distance from the axis of the designed 750 and 1150 kV overhead power lines to the boundaries of populated areas should, as a rule, be at least 250 and 300 m, respectively.
Household electrical appliances
The most powerful are microwave ovens, convection ovens, refrigerators with a “no frost” system, electric stoves, televisions, and computers. The actual EMF generated, depending on the specific model and mode of operation, can vary greatly among equipment of the same type. The electromagnetic field values are closely related to the power of the device. Moreover, the degree of pollution increases exponentially with increasing power.
Functional transmitters
Radar systems operate at frequencies from 500 MHz to 15 GHz, but individual systems can operate at frequencies up to 100 GHz. The EM signal they create is fundamentally different from radiation from other sources. This is due to the fact that periodic movement of the antenna in space leads to spatial intermittency of irradiation.
Temporary intermittency of irradiation is due to the cyclical operation of the radar on radiation. The operating time in various operating modes of radio equipment can range from several hours to a day. So, for meteorological radars with a time intermittency of 30 minutes - radiation, 30 minutes - pause, the total operating time does not exceed 12 hours, while airport radar stations in most cases operate around the clock.
The width of the radiation pattern in the horizontal plane is usually several degrees, and the duration of irradiation over the viewing period is tens of milliseconds. Meteorological radars can create a PES of ~100 W/m2 for each irradiation cycle at a distance of 1 km. Airport radar stations produce a PES of ~0.5 W/m2 at a distance of 60 m.
Marine radar equipment is installed on all ships; it usually has a transmitter power that is an order of magnitude lower than that of airfield radars, so in normal scanning mode the PES created at a distance of several meters does not exceed 10 W/m2. An increase in the power of radars for various purposes and the use of highly directional all-round antennas leads to a significant increase in the intensity of EMR in the microwave range and creates long-distance zones with a high energy flux density on the ground. The most unfavorable conditions are observed in residential areas of cities within which airports are located.
cellular
The main elements of a cellular communication system are base stations (BS) and mobile radiotelephones (MRT). Base stations maintain radio communication with mobile radiotelephones, as a result of which BS and MRI are sources of electromagnetic radiation.
An important feature of the cellular radio communication system is the very efficient use of the radio frequency spectrum allocated for the system’s operation (repeated use of the same frequencies, use of different access methods), which makes it possible to provide telephone communications to a significant number of subscribers.
The system uses the principle of dividing a certain territory into zones, or “cells,” with a radius of usually 0.5-10 kilometers. Base stations maintain communication with mobile radiotelephones located in their coverage area and operate in signal reception and transmission modes. Depending on the standard, BSs emit electromagnetic energy in the frequency range from 463 to 1880 MHz.[20] BS are a type of transmitting radio engineering objects, the radiation power of which (load) is not constant 24 hours a day.
The load is determined by the presence of cell phone owners in the service area of a particular base station and their desire to use the phone for a conversation, which, in turn, fundamentally depends on the time of day, location of the BS, day of the week, etc. At night, the load of the BS is almost zero .
A mobile radiotelephone (MRT) is a small-sized transceiver. Depending on the phone standard, transmission is carried out in the frequency range 453 – 1785 MHz. The MRI radiation power is a variable value that largely depends on the state of the communication channel “mobile radiotelephone - base station,” i.e., the higher the BS signal level at the receiving location, the lower the MRI radiation power.
The maximum power is in the range of 0.125-1 W, but in a real situation it usually does not exceed 0.05 - 0.2 W.
| Transceiver antennas on the roof of a residential building |
The question of the impact of MRI radiation on the user’s body still remains open. Numerous studies conducted by scientists from different countries, including Russia, on biological objects (including volunteers) have led to ambiguous, sometimes contradictory, results. The only undeniable fact is that the human body “responds” to the presence of cell phone radiation.
TV and radio stations
Television transmitters are usually located in cities. Transmitting antennas are usually located at altitudes above 110 m. From the point of view of assessing the impact on health, field levels at distances from several tens of meters to several kilometers are of interest. Typical electric field strengths can reach 15 V/m at a distance of 1 km from a 1 MW transmitter.
In Russia, at present, the problem of assessing the level of EMF of television transmitters is especially relevant due to the sharp increase in the number of television channels and transmitting stations. Transmitting radio centers (RTC) are located in specially designated areas and can occupy fairly large areas (up to 1000 hectares).
In their structure, they include one or more technical buildings where radio transmitters are located, and antenna fields on which up to several dozen antenna-feeder systems (AFS) are located. The AFS includes an antenna used to measure radio waves and a feed line that supplies high-frequency energy generated by the transmitter to it.
The zone of possible adverse effects of EMFs created by the PRC can be divided into two parts. The first part of the zone is the PRC territory itself, where all the services that ensure the operation of radio transmitters and AFS are located. This territory is guarded and only persons professionally associated with the maintenance of transmitters, switches and AFS are allowed into it.
The second part of the zone is the territories adjacent to the PRC, access to which is not limited and where various residential buildings can be located, in this case there is a threat of exposure to the population located in this part of the zone. The location of the PRC can be different, for example, in Moscow and St. Petersburg it is typically located in close proximity or among residential buildings.
Widespread sources of EMF in populated areas are currently radio engineering transmitting centers (RTTCs), emitting electromagnetic waves in the HF and UHF ranges into the environment.
Application
Infrared rays have been used to treat diseases since ancient times, when doctors used burning coals, hearths, heated iron, sand, salt, clay, etc.
to cure frostbite, ulcers, carbuncles, bruises, bruises, etc. Hippocrates described the method of using them to treat wounds, ulcers, damage from cold, etc. In 1894, Kellogg introduced electric incandescent lamps into therapy, after which infrared rays were successfully used for diseases of the lymphatic system, joints, chest (pleurisy), abdominal organs (enteritis, pain, etc.), liver and gall bladder. bubble In the infrared spectrum there is a region with wavelengths from approximately 7 to 14 microns (the so-called long-wave part of the infrared range), which has a truly unique beneficial effect on the human body. This part of the infrared radiation corresponds to the radiation of the human body itself, with a maximum at a wavelength of about 10 microns. Therefore, our body perceives any external radiation with such wavelengths as “our own.” The most famous natural source of infrared rays on our Earth is the Sun, and the most famous artificial source of long-wave infrared rays in Rus' is the Russian stove, and every person has definitely experienced themselves their beneficial influence.
Infrared diodes and photodiodes are widely used in remote controls, automation systems, security systems, some mobile phones, etc. Infrared rays do not distract human attention due to their invisibility.
Infrared emitters are used in industry for drying paint surfaces. The infrared drying method has significant advantages over the traditional convection method. First of all, this is, of course, an economic effect. The speed and energy consumed during infrared drying is less than the same indicators with traditional methods.
Infrared ray detectors are widely used by rescue services, for example, to detect living people under rubble after earthquakes or other natural and man-made disasters.
A positive side effect is also the sterilization of food products, increasing the corrosion resistance of painted surfaces.
A special feature of the use of IR radiation in the food industry is the possibility of penetration of an electromagnetic wave into capillary-porous products such as grain, cereals, flour, etc. to a depth of up to 7 mm. This value depends on the nature of the surface, structure, material properties and frequency characteristics of the radiation. An electromagnetic wave of a certain frequency range has not only a thermal, but also a biological effect on the product, helping to accelerate biochemical transformations in biological polymers (starch, protein, lipids)
Effect of EMR on humans
It is believed that electromagnetic radiation has a negative impact on both human health and his behavior, vitality, physiological functions and even thoughts. The person himself is also a source of such radiation, and if other, more intense sources begin to influence our electromagnetic field, then complete chaos can occur in the human body, which will lead to various diseases.
Scientists have found that it is not the waves themselves that are harmful, but their torsion (information) component, which is present in any electromagnetic radiation, that is, it is the torsion fields that have the wrong effect on health, transmitting negative information to a person.
The danger of radiation also lies in the fact that it can accumulate in the human body, and if you use, for example, a computer, mobile phone, etc. for a long time, then headaches, high fatigue, constant stress, decreased immunity are possible, and the likelihood of diseases of the nervous system and brain. Even weak fields, especially those that coincide in frequency with human EMR, can harm health by distorting our own radiation, and thereby causing various diseases.
Electromagnetic radiation factors have a huge impact on human health, such as:
- source power and nature of radiation;
- its intensity;
- duration of exposure.
It is also worth noting that exposure to radiation can be general or local. That is, if you take a mobile phone, it affects only a separate human organ - the brain, but the radar irradiates the entire body.
What kind of radiation arises from certain household appliances, and their range, can be seen from the figure.
Looking at this table, you can understand for yourself that the further the radiation source is located from a person, the less its harmful effect on the body. If a hairdryer is in close proximity to the head, and its impact causes significant harm to a person, then the refrigerator has practically no effect on our health.
Exposure to meter waves
High-intensity meter waves emitted by pulse generators of meter radar stations (radars) with a pulse power of more than a megawatt (such as the P-16 early warning station) and commensurate with the length of the spinal cord of humans and animals, as well as the length of axons, disrupt conductivity these structures, causing diencephalic syndrome (HF disease).
The latter leads to the rapid development (over a period of several months to several years) of complete or partial (depending on the received pulse dose of radiation) irreversible paralysis of a person’s limbs, as well as disruption of the innervation of the intestines and other internal organs.
Impact of decimeter waves
Decimeter waves are comparable in wavelength to blood vessels, covering such human and animal organs as the lungs, liver and kidneys. This is one of the reasons why they cause the development of “benign” tumors (cysts) in these organs. Developing on the surface of blood vessels, these tumors lead to the cessation of normal blood circulation and disruption of organ function.
If such tumors are not surgically removed in time, the death of the body occurs. Decimeter waves of dangerous intensity levels are emitted by the magnetrons of such radars as the P-15 mobile air defense radar, as well as the radar of some aircraft.
Exposure to centimeter waves
Powerful centimeter waves cause diseases such as leukemia - “white blood”, as well as other forms of malignant tumors in humans and animals. Waves of intensity sufficient for the occurrence of these diseases are generated by the centimeter range radars P-35, P-37 and almost all aircraft radars.
Instruments for measuring electromagnetic radiation (EMR)
Today there are many different instruments for measuring electromagnetic radiation. Among them there are both very expensive universal models and fairly budget-friendly, simplified modifications. There is a widespread opinion on thematic forums that it is advisable to use the latter to independently measure the magnetic field. This is a pretty serious misconception, and here's why:
- To obtain correct measurement results, it is necessary to strictly comply with all relevant SanPin requirements. Depending on the research method and location, they vary significantly.
- the optimal research method can be selected based on data on the level of energy concentration, magnetic field intensity and wave frequency range. To do this you need to have special knowledge. Otherwise, the measurement results will be rather approximate.
- Each device has a special operating algorithm. To use the device correctly and effectively, you also need to have special knowledge and experience.
- Any device for measuring EMR must be certified, and the person conducting the research must have a license for this field of activity.
Therefore, EMF measurements should be entrusted to specialists.
How to protect yourself from electromagnetic radiation
The danger of EMR lies in the fact that a person does not feel its influence in any way, but it exists and greatly harms our health. While workplaces have special protective equipment, things are much worse at home.
But it is still possible to protect yourself and your loved ones from the harmful effects of household appliances if you follow simple recommendations:
- purchase a dosimeter that determines the intensity of radiation and measure the background from various household appliances;
- do not turn on several electrical appliances at once;
- keep your distance from them if possible;
- place devices so that they are located as far as possible from places where people spend a long time, for example, a dining table or a recreation area;
- children's rooms should contain as few radiation sources as possible;
- there is no need to group electrical appliances in one place;
- The mobile phone should not be brought closer to the ear than 2.5 cm;
- Keep the telephone base away from the bedroom or desk:
- do not be located close to a TV or computer monitor;
- turn off devices you don't need. If you are not currently using a computer or TV, you do not need to keep them turned on;
- try to reduce the time you use the device, do not stay near it all the time.
Modern technology has firmly entered our everyday life. We cannot imagine life without a mobile phone or computer, as well as a microwave oven, which many have not only at home, but also in the workplace. It’s unlikely that anyone will want to give them up, but it’s within our power to use them wisely.
What disorders does electromagnetic radiation cause?
Under the influence of radiation harmful to the body, people often develop disorders of the central nervous system. Typical EMF victims suffer:
- insomnia;
- neurasthenia;
- depressive disorders;
- increased anxiety;
- headaches;
- rapid fatigue;
- asthenic syndrome.
Those whose homes are crammed with electronic and electrical appliances experience deteriorating memory, reduced resistance to stress, and often experience mood swings, apathy, or states of agitation.
Typical disorders of the vascular system:
- bradycardia or tachycardia;
- regular increase in heart rate;
- dyspnea;
- pressing dull pain in the heart area;
- increased sweating;
- feeling of heat in the body;
- surges in blood pressure.
In hypotensive patients and those suffering from hypertension, exacerbations of pathological conditions near radiation sources occur more often than in “clean” territory. Leukocytosis and a decrease in the number of red blood cells in the blood are sometimes observed.
Due to decreased pituitary function, the endocrine system is likely to deteriorate. Under the influence of EMF, adrenaline and cortisol are more actively released, and the synthesis of sex hormones decreases. It has been established that aggressive electromagnetic radiation aggravates infectious and inflammatory processes, inhibits cellular immunity, reducing resistance to various external pathogens.
There is evidence that some types of radiation can exhibit teratogenic properties. For pregnant women, this risks premature abortion, critical disturbances in the development of the fetus, and the formation of congenital deformities or serious illnesses in the child. In men, intensive work with electromagnetic equipment is fraught with impaired spermatogenesis and decreased libido.
Practical application of protection methods
Solving home problems related to exposure to electromagnetic fields should begin with a simple check. To do this, it is necessary to determine the level of magnetic and electric field strength in an apartment or house. If the indicators do not go beyond the maximum permissible levels that were discussed, then do not worry, they are calculated with a multiple margin.
If there is a problem, then proven methods are used to reduce the impact of electromagnetic waves:
- Check the presence and connection of sockets to grounding circuits. It is recommended to use these elements with special PE conductor contacts.
- Microwaves and other potentially dangerous household devices are equipped with housings with protective shielding. Operation is not allowed even in a partially disassembled state.
- Stationary equipment must be grounded, for this reason it is important to have sockets with appropriate contacts.
Among other well-known methods of radiation protection, we recommend that possible sources be located at the greatest possible distance. You should not sleep next to a microwave, and it is better to use a mobile phone with a headset. But these are common truths, so we won’t dwell on them.
Let us remind you once again that you should only worry about the effects of electromagnetic radiation if an instrumental test has revealed an increased level of field strength. An apartment saturated with electrical appliances is not a reason to panic; given acceptable standards, there is no threat to health. A tin foil hat can only be used as an extravagant accessory.
Sources
- https://ObOtravlenii.ru/izluchenie/elektromagnitnoe/elektromagnitnoe-izluchenie.html
- https://autogear.ru/article/417/078/fizika-protsessa-izlucheniya-primeryi-izlucheniya-v-byitu-i-prirode/
- https://fizmat.by/kursy/jelektromagnt/jelmagn_volny
- https://alfapol.ru/istochniki-elektromagnitnogo-izlucheniya-vokrug-nas/
- https://electricity-help.ru/dolzhen-znat-kazhdyy/yelektromagnitnoe-izluchenie/
- https://bourabai.ru/toe/em-scale.htm
- https://OFaze.ru/teoriya/zashhita-ot-elektromagnitnogo-izlucheniya
[collapse]