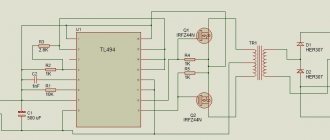
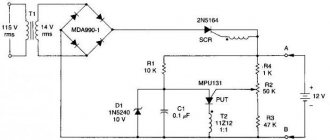
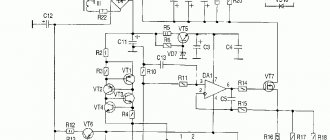
A battery is a chemical source of electrical voltage. All commercially available batteries have similar operating principles. The positive terminal of the product is made of manganese or lithium, the negative terminal is made of zinc or aluminum. You can assemble the battery yourself from simple materials.
Batteries are a source of electrical voltage.
Homemade battery from improvised means
The battery can be made from materials whose properties are similar to those of substances used in industrial conditions.
From lemon
The acid contained in the juice of the fruit acts as an electrolyte. Electrodes are made from thin wire, nails or needles. The iron element is the anode, the copper element is the cathode. The lemon is cut in half and placed in a small container (jar or glass). The wires are connected to the electrodes, the stripped ends are inserted into the pulp of the fruit at a distance of 1 cm from each other.
Using a multimeter, measure the voltage supplied by a homemade galvanic cell. If it is not high enough, several lemon batteries are connected in series.
Jar with electrolyte
Using this method, it is possible to assemble a device that resembles the world's first battery. Electrodes are made of copper and aluminum. Elements must have a large area. The aluminum electrode is connected to the wire using a clamp or bolt, while the copper electrode is soldered. The parts are immersed in a jar at a short distance from each other. A lid with holes is used for fixation. The following compositions are used as electrolytes:
Making batteries with your own hands.
- Ammonia. The substance is mixed with water in a ratio of 1:2. Ammonia cannot be used as an electrolyte. A suitable substance (ammonium chloride) has the form of a white, odorless powder. It is used as a fertilizer or soldering flux.
- Sulfuric acid solution. The substance is mixed with water in a ratio of 1:5. You cannot pour the acid first. In this case, the added water boils and splashes fall on the person’s skin and clothing.
The solution is poured into a glass container so that the distance to the edges of the jar is at least 2 mm. Using a multimeter, measure the resistance and calculate the required number of batteries. The principle of operation of a homemade element is similar to that of a salt power source.
Copper coins
The electrodes are made of aluminum and copper; 9% acetic acid is used as the electrolyte. Coins are cleaned of dirt by soaking them in vinegar. Circles are cut out of cardboard and foil. Cardboard products are soaked in a solution of acetic acid; they must absorb the electrolyte. A column is laid out from circles and coins.
The cardboard piece is placed first, the second is made of foil, and the third is a coin. Wires are pre-connected to the extreme elements. Instead of soldering, the cables can be pressed to metal parts and sealed with tape. When the battery is used, the coin becomes unusable. You should not make power supplies from valuable products.
Battery in a beer can
The negative terminal is the body of the aluminum container, the positive terminal is the graphite rod. You will also need coal dust, polystyrene foam, water, paraffin candles and salt. The top of the jar is removed, a circle is cut out of foam plastic, which is inserted into the container. A hole for the rod is made in advance. The latter is installed in the central part of the jar. The remaining space is filled with coal dust. The material is impregnated with an aqueous solution of salt (3 tablespoons of product per 0.5 liters of water). The edges of the jar are filled with paraffin.
Potatoes, salt and toothpaste
The potato battery is intended for one-time use. It is used to produce a spark by shorting wires. To make the element you will need a large potato, insulated copper cables, salt, wooden sticks and toothpaste. The assembly is done like this:
- Potatoes are cut into 2 equal parts. A recess is formed in one half, where salt and paste are added.
- The ingredients are mixed until smooth. The electrolyte should fill the depression.
- In the other half of the potato, make 2 holes at a distance of 1-2 cm. They should coincide with the filled recess.
- The stripped ends of the wires are inserted into the holes, and the halves are combined. The wires must be immersed in the composition.
- Potato parts are secured with toothpicks. After a few minutes, the cables short out, creating a spark to start the fire.
, you need to call or do something else. So, let's
let's make a battery
from what we have on hand!
1. Battery from saline solution
To make a galvanic cell, we need:
1) A large vessel (a bucket, maybe even a holey one, or something like that, you can even use plastic bags) 2) A zinc and copper plate. If there are no plates, then you can simply use zinc and copper wire, but the plates have a larger area and produce more current. 3) Earth. Yes, you can just dig up some soil. 4) Saline solution. I won’t give any exact recommendations here. Half a pack of salt is enough for a bucket of water.
It’s simple - we fill it with soil, stick in the electrodes, water it, and at the ends of the electrodes you will see a voltage of about 0.5-1V. Of course, not much, but what’s stopping you from making a battery of such elements? Enough to charge a mobile phone. Pour it in, pour it in and go about your business!
A good option for a homemade element is an air-aluminum one. To do this, you need to take aluminum cathode foil, soak a napkin with salt (or sea water), I also tried using acidic flux, a pile of carbon powder as an anode, I took toner from laser printer cartridges. The voltage is 0.5-1.0V at a current of 10mA
2. Battery made from fruits and vegetables
To make a galvanic cell we need: two electrodes, an oxidizing agent, a reducing agent and an electrolyte. Let's take three plates: copper, iron and magnesium - they will serve as electrodes. To measure voltage, we need a voltmeter; a digital (or analog) tester is quite suitable for these purposes. And as a “glass” with electrolyte we use a large and beautiful... orange. Fruit and vegetable juice contains dissolved electrolytes - salts and organic acids. Their concentration is not very high, but that suits us quite well.
So, let's put an orange on the table and stick our three electrodes (copper, iron and magnesium) into it. Pre-attach a wire to each of the electrodes (it’s convenient to use alligator clips for this). Now connect the tester contacts to the copper and iron electrode. The device will show a voltage of about 0.4-0.5 V. Disconnect the contact from the iron electrode and connect it to the magnesium one. Between the copper and magnesium electrodes there will be a potential difference of about 1.4-1.5 V - approximately the same as that of a finger-type battery. And finally, the iron-magnesium galvanic cell will give a voltage of about 0.8-0.9 V. If you swap the contacts, the sign of the device will change (“+” to “-” or vice versa). In other words, current will flow through the voltmeter in the opposite direction.
Instead of an orange, you can use grapefruit, apple, lemon, onion, potato and many other fruits and vegetables. It is curious that batteries made from orange, apple, grapefruit and onion gave fairly close voltage values - the difference did not exceed 0.1 V. The reducing agent in our case is iron or magnesium, the oxidizing agent is hydrogen ions and oxygen (which are contained in the juice). Note that the iron in a copper-iron cell is negatively charged, while the iron in an iron-magnesium cell is positively charged. If you do not have magnesium, the experiment can be carried out with two electrodes - copper and iron. Instead of iron, you can take zinc or a piece of galvanized sheet. A zinc electrode should give a larger potential difference with copper and a smaller one with magnesium.
In the case of citrus fruits, the experiment looks especially beautiful if you cut the fruit crosswise so that the “slices” are visible and insert electrodes into them (usually this is how a lemon is cut). If the fruit is cut lengthwise, it will not look so impressive.
The figures given should not be taken as absolute. The voltage of our battery depends on the concentration of hydrogen ions (as well as other ions) in the juice of fruits and vegetables, the rate of oxygen diffusion, the condition of the surface of the electrodes and other factors. The voltage of the battery you make may differ significantly from what was observed in this experiment. You can connect several fruit batteries in series - this will increase the voltage in proportion to the number of fruits taken.
The same materials are suitable for a potato battery, but it produces less voltage, so it is recommended to add a little salt inside the potato, the effect will be much greater.
3. Coffee battery (Nespresso battery)
In an effort to show the world the importance of collecting and recycling valuable aluminum materials, Vienna-based designers at Mischer' Traxler have developed batteries from 700 used aluminum cans and coffee grounds to power a quartz watch.
The design developed was called the “Nespresso Battery”, the installation is made from old aluminum cans, coffee grounds, strips of copper and salt water. In the photo below:
- a clock as a testing device - salt - ground coffee - wires - copper plates - aluminum plates - glass - plastic bottle separator
In a glass we put a copper plate (textolite, coin, thick wire) and aluminum slices (from beer cans). To prevent copper and aluminum from coming into contact, we place a separator between them made of any dielectric (plastic from a bottle, coffee grounds), and it should not interfere with the free flow of water. We connect wires to the plates, one to copper and one to aluminum. Now take water and add a few tablespoons of salt there, mix them until the salt is completely dissolved. Pour this solution into a glass. The battery is all done.
Each battery produces enough energy for an electromechanical quartz watch. And 17 batteries are enough to operate a small radio. The device is one of three winners in a competition entitled "Sustainability by Design".
The coffee grounds here are purely for decoration, and so that you can give it a nice name. And so its function can be used to separate conductors, you can completely abandon coffee grounds.
4. Baghdad battery (Parthian battery)
A small Parthian vessel was found at Khuzhut Rabu, in the vicinity of modern Baghdad (now Iraq), once part of the western territories of Greater Iran. In June 1936, a new railway was being laid near Baghdad - and workers discovered an ancient burial place. Subsequent excavations revealed that it belongs to the Parthian period (c. 250 BC - 250 AD).
One of the finds was a clay vessel with an asphalt “stopper”. An iron rod passed through the “plug”. Inside the vessel, the rod was lowered into a copper cylinder. This vessel was first described by the German archaeologist Wilhelm Koenig in 1938 - he considered it very similar to an electric battery, and published an article on this topic in 1940.
Using a similar principle, you can assemble your own battery. We take a “vessel” that can be made from: clay, plasticine, a bottle, a jar, a glass, insert into it a copper plate twisted into a cylinder, and insert a nickel-plated nail into this cylinder. These plate and nail are electrodes, they should stick out a little from the can. To secure them in the body of the “vessel” you can use: epoxy glue, plasticine, window putty, etc. Now we need to make an electrolyte. It can be alkaline or acidic. For alkali, you need to make a concentrated solution of: water + salt or water + soda. For acidic, diluted acetic or oxalic acid in water is suitable, or you can use citrus juice.
Pour the electrolyte inside the jar and carefully seal the “vessel”. The Baghdad battery is ready.
When such a model is filled with electrolyte, it can produce voltage. In general, depending on the type of electrolyte, the voltage supplied by the “battery” varies from 0.5 to 2 volts.
Unfortunately, due to the destruction of many Iranian literary sources and libraries during enemy invasions of Iran over the centuries, there are no written records of what exactly such vessels were used for. Everything we know about them today is just guesswork.
5. Solar battery
Having read on the endless expanses of the Internet about homemade solar cells, I decided to conduct my own “experiments” in this area. I will tell you about the easiest way to make solar panels with your own hands.
To begin with, I decided to decide on the element base. For a solar cell we need PN junctions. They are found in diodes and transistors. It was decided to choose KT801 silicon transistors. They were produced in a metal case and therefore they can be opened without damaging the crystal. It is enough to press the lid with pliers and it will break off.
Now let's look at the parameters. In average daylight, each of our transistors produces 0.53V (Base is plus, and Collector and Emitter are minuses). And then there is one nuance. Transistors from 1972 have a large white crystal, and produce about 1.1mA. Transistors from 1973 to 1980 The releases have a large crystal with a green coating, and produce about 0.9mA. Transistors released later have small crystals and produce only 0.13mA.
For the experiment, I used a battery of two parallel chains of 4 transistors. Under load it produced about 1.8V, 2-2.5mA. These are rather modest parameters, but as they say, “for free”. You can power a Chinese wristwatch with such a battery, or charge the battery and power an LED, bug, etc.
For ease of mounting and measurements, you can mount the transistors on a printed circuit board as in the figure below. My device is wall-mounted, as this speeds up assembly.
6. Coin-energy battery
It seems that the design is standard, zinc-copper contacts and salted water, but the design of the battery itself is interesting.
We will need:
- ice tray - copper/copper alloy coins - nickel/aluminum bronze/zinc coins - paper clips - salt - water - LED (for checking)
To get a battery, you need to connect coins into electrodes and fill them with electrolyte. In each cell of the tray you need to place two coins from different alloys, for example copper and nickel. Next, we connect all the cells in series using a paper clip. Pressing a copper coin to one side of the wall and a nickel coin to the other, we secure them with a paper clip. After this, you need to fill each tray with electrolyte: salt + water. Pay attention to the ends of the tray, since the cells are in two rows, on one side we need to connect them, and on the other they should remain unconnected. Now we check the performance of the battery using a diode or a multimeter; to do this, we close two unconnected cells with it.
One cell produces electricity with a voltage of 0.5 V, and those connected into one battery produce 2 V and 110 mA. Therefore, it is desirable to have one electrolyte for all cells, and not heterogeneous ones.
Peculiarities:
1. The cell should be completely filled with electrolyte, but contact should only be with a coin, not a paper clip. 2. One of the pairs of cells should not be shorted to each other. 3. Zinc coins are used as positive electrodes, and copper coins are used as negative electrodes. 4. Coins must be made of different metals/alloys (copper and nickel), it is also desirable that they do not contain the same impurities in the alloys.
7. Homemade battery
Now we will make a fairly simple device, or rather a power source - a homemade voltage battery. As is known, two different metals immersed in an electrolyte solution are capable of accumulating electric current. It was decided to use copper and aluminum foil as electrodes (in my opinion, they are the most affordable).
In addition to foil, we also need a sheet of paper, transparent tape and the vessel itself in which we will place the battery jar (it is very convenient to use a glass vessel made from naphthysine or valerian tablets).
Let's look at the photographs.
The foils are almost the same size, only the aluminum foil is a little longer, there is no reason for this, it is simply easier to apply solder to copper foil than to aluminum foil and the wire is not soldered to the foil, it is simply rolled into it and then clamped with pliers.
Next, both foils were wrapped in a sheet of paper. It is not permissible for metals to touch each other; a sheet of paper serves as a barrier between them. Then the foils need to be taken together and wrapped in a circle and wrapped with thread or transparent tape.
Then the completed package must be placed in a vessel. After this, take 50 ml of water and dilute 10 - 20 grams of salt into it. Mix the solution thoroughly and heat until all the salt has melted.
After melting the salt, pour the solution into a vessel where we have a ready-made blank for our homemade battery. After filling, wait a few minutes and measure the voltage on the battery wires. I forgot to specify the polarity of the battery, copper foil is a plus for power supply, aluminum foil is a minus. Measurements will show a voltage of the order of 0.5-0.7 volts. But the initial tension doesn't mean anything. We need to charge our battery. You can charge from any DC source with a voltage of 2.5-3 volts, charging lasts half an hour. After charging, we measure the voltage again, it increased to 1.3 volts and can reach up to 1.45 volts. The maximum current of such a homemade battery can reach up to 350 milliamps.
You can make several of these batteries and use them as a backup power source for, say, an LED panel or flashlight. To increase the power of the battery, you can use large foil, but of course such a homemade battery will not hold a charge for very long (the charge will run out within one week), another disadvantage is the short service life (no more than 3 months), since oxide forms on copper During the charge-discharge process, the aluminum foil begins to corrode and will gradually separate into small pieces, but I think for experiments it’s worth trying to assemble such a simple battery.
8. DC adapter
Having a little free time and desire, it’s easy to assemble an adapter from scrap materials to power various gadgets from an external power source.
What I liked about this article is the simplicity of this adapter. I will describe the manufacturing technology in more detail. I think it will be useful to someone else, especially since there is absolutely nothing complicated here. I didn’t even go anywhere to get the material. There was an old MTS card lying on the table. It was not in vain that he paid a hundred rubles. I tried it on, it’s exactly suitable for making a model of one battery for a camera. Cutting cardboard:
There are even very few scraps left.
The cardboard is what you need - hard, about 0.25 mm thick. I made markings and cut along the seams. The cardboard was not cut all the way through, but about a little more than half the thickness, to make bending and gluing easier. For contacts, I riveted 1.5 square millimeter copper wire. It turned out something like this.
This is what the contacts look like from the inside:
I soldered the wires and double-glued all the seams with Moment STOLYAR PVA glue. The seams are thin, so I had to smear them patiently, drop by drop, with the tip of a toothpick... Although, if you can’t wait, you can glue them together with tape. We connect to the “vampire” and work:
Connected it, everything worked.
So far, only one inconvenience has been discovered - the wire. He’s fat, reaching for the camera and the “vampire little one.” Therefore, I decided to attach to the camera the same battery as in the “vampire little one,” only with protection. By the way, it is not necessary to install batteries with protection here, because... The camera already has a built-in charge level meter and if the battery is low it simply will not turn on.
And don't forget to observe polarity!!!
>
Random site materials
Cleaning siphon 6935
Required materials and tools
Before starting the experiment, prepare the following materials and tools:
- corrugated cardboard;
- flat copper washers with a diameter of 1 cm - 12 pcs.;
- flat zinc washers with a diameter of 1 cm – 15 pcs.;
- purified water;
- heat-shrink tubing;
- acetic acid 70%;
- salt;
- soldering iron;
- containers for preparing solutions;
- multimeter;
- sandpaper.
Corrugated cardboard is one of the materials for making batteries yourself.
Potato battery
- two potatoes;
- three wires with clamps;
- two chrome nails;
- two copper nails.
So, how to make a battery from tubers:
- Give a symbol for each of the potatoes - “A” and “B”.
- Insert a chrome nail into the edges of each tuber.
- On the opposite edge - a copper nail. The nails should not intersect in the body of the potatoes.
- Take any battery-powered device, remove it, and leave the compartment open.
- The first wire should connect the copper pin of the tuber "A" to the positive terminal in the battery compartment.
- The second wire connects the chrome pin of potato "B" to the negative terminal.
- The last wire connects the chrome tuber nail "A" to the copper tuber nail "B".
- As soon as you close all the wires in this way, the potato will begin to supply energy to the device.
Potatoes in this experiment can be replaced with banana, avocado or any of the citrus fruits.
Coin battery
A structure made of coins as the simplest galvanic element is also called a Voltaic column. To make it you will need:
- copper coins (50 or 10 kopecks);
- foil;
- paper;
- vinegar or very salty water.
For the beauty of the design, it is necessary to choose coins of the same denomination. Also, before the experiment, dip them briefly in vinegar. This will remove plaque and dirt. Then you need to cut out elements in the shape of coins from paper and foil. Their number should be 2 less than coins.
The voltaic column is assembled like this:
- The paper is soaked in a solution of vinegar or salt water and attached to a coin.
- A circle of foil is placed on top of the paper.
- Next, the next coin is placed.
- The stages are repeated until the selected amount of coins runs out.
- The design should be such that at one end there is a coin (+) as the last element, and at the other end there is foil (-).
The more coins are used in the experiment, the more voltage the battery will produce. It is important to understand that after the experiment the coins will not be suitable for use. Elements become covered with rust.
The charge occurs due to the fact that an electrolyte (vinegar or saline solution) placed between two metals (foil and coins) creates a potential difference.