A mixture of fluorine and sulfur, known as SF6 gas, is widely used as the main insulator in electrical installations. At normal temperature and operating pressure, it is colorless and odorless, non-flammable and almost 5 times denser and heavier than air. The properties of SF6 gas remain unchanged for an unlimited time. When an electrical discharge enters its environment, it first disintegrates and then quickly restores its original dielectric strength. Due to its properties, SF6 gas is used in gas-insulated arc extinguishing devices and is the basis of SF6 insulation.
Physical and chemical nature of SF6 gas
From a chemical point of view, SF6 gas is an extremely inert compound. It does not react to acids and alkalis, oxidizing agents and reducing agents. This substance has increased resistance to molten metals, is slightly soluble in water and interacts only with organic solvents.
To decompose this compound, a temperature of 1100 degrees or higher is required. The decomposition products are gaseous components that have toxicity and a specific pungent odor. Accumulating indoors, SF6 gas can cause oxygen deficiency. In general, it is a low-hazard substance with a maximum permissible concentration indoors of 5000 mg/m3, and in the open air of 0.001 mg/m3.
When a compound captures electrons, low-mobility ions are formed. As a result, the number of charge carriers is significantly reduced. Their acceleration in an electric field is extremely slow, which prevents the formation and development of electron avalanches. Due to this, SF6 gas has high electrical strength. Increased pressure promotes an increase in electrical strength in proportion to the applied pressure. Often this indicator exceeds the same parameter for liquid and solid dielectric materials.
A significant disadvantage of SF6 gas is the loss of its insulating qualities and the transition to a liquid state under the influence of low temperatures. Therefore, additional requirements are imposed on the temperature regime of SF6 installations. One of the most suitable options for solving such situations is mixing SF6 gas with other types of gases, for example, nitrogen. Another method is to use a heating system, which significantly increases the reliability of equipment at temperatures of minus 40 and below.
Application[ | ]
- as an insulator and coolant in high-voltage electrical engineering;
- as a technological environment in the electronics and metallurgical industries;
- in gas fire extinguishing systems as a fire extinguishing agent;
- as a refrigerant due to its high heat capacity, low thermal conductivity and low viscosity[5];
- to improve sound insulation in double-glazed windows;
- in the semiconductor industry for plasma-chemical etching of silicon;
- as an oxidizer in some exotic heat engines - for example, in the steam turbine unit of the American small-sized 324-mm Mark 50 anti-submarine torpedo, where it is used to oxidize lithium metal.
When inhaled, a lowered voice effect is observed, which is the opposite of the effect of helium[6].
Application in electrical engineering[ | ]
Sulfur hexafluoride gets its name “sulfur hexafluoride” from the abbreviation “electric gas”. The unique properties of SF6 gas were discovered in the USSR, and its use also began in the Soviet Union. In the 30s, the famous scientist B. M. Gokhberg at the Leningrad Physics and Technology Institute studied the electrical properties of a number of gases and drew attention to some properties of sulfur hexafluoride SF6 (SF6 gas) [7]. The need for SF6 gas appeared in the country in the early 1980s and was associated with the development and development of electrical equipment for ultra-high voltage direct current transmissions. Its industrial production in the Russian Federation was mastered in 1998 at the Kirovo-Chepetsk Chemical Plant[8].
The electrical strength at atmospheric pressure and a gap of 1 cm is 89 kV/cm. Characteristic is a very large coefficient of thermal expansion and high density. This is important for power plants in which any parts of the device are cooled, since with a large coefficient of thermal expansion, a convective flow easily forms, carrying away heat [9].
At the center of the SF6 gas molecule there is a sulfur atom, and at an equal distance from it at the vertices of a regular octahedron there are six fluorine atoms. This determines the high efficiency of electron capture by molecules, their relatively long free path and weak reactivity. Therefore, SF6 gas has high electrical strength.
SF6 gas is harmless when mixed with air. However, due to a violation of the SF6 gas production technology or its decomposition in the apparatus under the influence of electrical discharges (arc, corona, partial), extremely chemically active and harmful impurities for humans, as well as various solid compounds deposited on the walls of the structure, may appear in the SF6 gas. The intensity of the formation of such impurities depends on the presence of oxygen impurities and especially water vapor in the SF6 gas.
Some SF6 gas in electrical equipment also decomposes during normal operation. For example, switching a current of 31.5 kA in a 110 kV switch leads to the decomposition of 5-7 cm³ of SF6 gas per 1 kJ of energy released in the arc.
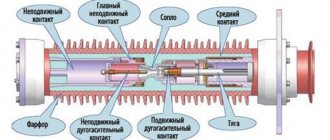
The cost of SF6 gas is quite high, but it has found quite wide application in technology, especially in high-voltage electrical engineering. It is primarily used as a dielectric, that is, as the main insulation for complete switchgears, high-voltage current and voltage instrument transformers, etc.[10]. SF6 gas is also used as an arc extinguishing medium in high-voltage circuit breakers[11].
The main advantages of SF6 gas over its main “competitor”, transformer oil, are:
- explosion and fire safety;
- reducing the weight and dimensions of the structure due to reduced insulation gaps and improved cooling conditions for live parts [ source not specified 2910 days
].
Regulatory Standards[ | ]
IEC
- IEC 60376:2005 - Specification for technical grade SF6 gas for electrical equipment.
- IEC 60480:2004 - Guidelines for the testing and treatment of sulfur hexafluoride (SF6) from electrical equipment and specifications for its reuse.
[en]
- EN 60376:2005 - Specification for technical grade SF6 gas for electrical equipment.
- EN 60480:2004 - Guidelines for the testing and treatment of SF6 gas from electrical equipment and specifications for its reuse.
Harmful effects[ | ]
Main source: [12]
In terms of the degree of impact on the human body, it is classified as a low-hazard chemical substance (hazard class IV according to GOST 12.1.007-76).
There is a possibility of poisoning by SF6 decomposition products (lower fluorides), which are formed, for example, during the operation of arc chutes in high-voltage circuit breakers.
Ozone depletion potential ODP = 0.
The strongest known greenhouse gas, global warming potential GWP = 24,900. Due to small production volumes, the contribution to global warming does not exceed 0.2%. Regulated by the Kyoto Protocol.
Arcing properties of SF6 gas
Under all the same conditions, SF6 gas has a significantly greater arc extinguishing ability compared to ordinary air. The main factors are the plasma composition, SF6 gas density, as well as heat capacity, thermal and electrical conductivity, which are temperature dependent.
When the plasma state is reached, the SF6 molecules disintegrate. When the temperature reaches 2000 K, there is a sharp increase in heat capacity due to molecular dissociation. Therefore, in the temperature range between 2000 and 3000 K, the thermal conductivity of plasma increases many times compared to ordinary air. When the temperature reaches 4000 K, the dissociation of molecules begins to decrease.
At the same time, atomic sulfur is formed in the SF6 arc. Its low ionization potential causes a concentration of electrons that is capable of maintaining an arc even at a temperature of 3000 K. A further increase in temperature leads to a drop in the thermal conductivity of the plasma, as a result, this parameter becomes the same as that of air. Then the thermal conductivity increases again.
Due to these processes, the resistance and voltage of the burning arc in SF6 gas is reduced by approximately 20-30% relative to the arc occurring in air. This state is maintained up to temperatures from 8 to 12 thousand degrees. When the plasma temperature begins to decrease to 7000 K and further, the electron concentration in it correspondingly decreases, which leads to a drop in the electrical conductivity of the plasma.
Characteristics of SF6 gas, main advantages
Main properties of SF6 gas:
- significant specific heat capacity, four times higher than that of air, which makes it possible to reduce the amount of copper in equipment and increase the load on live parts;
- electrical strength exceeds atmospheric indicators by 2.5 times; if we talk about a pressure of 0.2 MPa, then the gas becomes close in quality to transformer oil;
- with SF6 gas, the rated shutdown current of the longitudinal blast chamber is 5 times higher compared to air;
- there is low electric field strength in the arc column; this helps reduce blockage by reducing the thermodynamic effect and helps increase the distance between contacts;
- SF6 gas does not react with oxygen because it is an inert substance; it is weakly decomposed by an arc;
- the compound is non-toxic; Decomposition products can be dangerous, but this is rare.
Among the disadvantages is the high reduction temperature. This does not matter when creating equipment operating at 35 kV or more. SF6 gas is widely used today in the manufacture of switching equipment, including circuit breakers and contactors. Such devices are widely used in all power distribution schemes.
Industrial production of SF6 gas
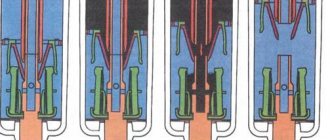
The industrial method for producing SF6 gas is based on a direct reaction between fluorine gas and molten sulfur. In this case, sulfur is burned in a fluorine stream at a temperature of 138-149C in a special cracking furnace, which is a steel horizontal reactor. This device consists of a loading chamber and a combustion chamber, separated by a partition. The loading chamber is equipped with a hatch through which sulfur is loaded and an electric heater for melting.
The combustion chamber has a water-cooled nozzle through which fluorine is supplied. A thermocouple and a condenser for sublimation of sulfur are also installed here. The sulfur itself, in molten form, is fed from the loading chamber into the combustion chamber through a special hole located in the lower part of the partition. The hole is closed with molten sulfur, which prevents fluorine from entering the loading chamber.
This reactor, despite its simple design, has some negative qualities. Sulfur is fluorinated on the surface of the melt, which causes heat to be released in large quantities. Under its influence, as well as under the influence of fluorine, increased corrosion of the reactor occurs at the separation boundary of the production cycle. Therefore, when the productivity of the reactor increases, it becomes necessary to remove large amounts of heat and select a corrosion-resistant reactor material.
SF6 gas production
SF6 gas is produced in the following ways:
- - the main industrial method of production: as a result of a direct reaction between molten sulfur and gaseous fluorine obtained through its electrolysis (combustion of sulfur in a fluorine flow - Fig. 2). The reaction takes place at a temperature of 138 - 149 °C in a steel horizontal reactor (cracking furnace). The reactor is divided by a partition into a loading chamber and a combustion chamber. The loading chamber has a hatch for loading sulfur and an electric heater for melting it. The combustion chamber has a nozzle for supplying fluorine, cooled by water, a thermocouple and a condenser for sublimation of sulfur located above the chamber. The sulfur melt flows from the loading chamber into the combustion chamber through an opening at the bottom of the partition, closed by the melt, which prevents fluorine from escaping into the loading chamber. Despite its simplicity, this reactor design has some disadvantages, namely: sulfur is fluorinated on the surface of the melt with the release of a large amount of heat, which causes increased corrosion of the reactor by fluorine at the interface;
- when the reactor productivity increases, the problem of removing a large amount of heat and selecting a corrosion-resistant reactor material arises;
- Another disadvantage of the method is that during this synthesis of SF6 gas, other fluorides are simultaneously formed - S2F2, SF2, SF4, and S2F10, as well as impurities due to the presence of moisture, air and carbon anodes used for fluorine electrolysis. The concentration of these substances is low, averaging 0.01 - 0.1% by volume. But if chemically pure SF6 gas is non-toxic and is a very inert compound that does not react with any materials up to a temperature of 300 ° C, then impurities can change the mentioned properties of the product and even make it unsuitable for use. Therefore, careful cleaning of the produced SF6 gas is necessary. The composition of pure SF6 gas is regulated by TU 6-02-2-686-82 and the IEC 6o 376 standard (the absence of toxic impurities occurring in its production technology is guaranteed by the manufacturer based on biological control of the batch);
Rice. 2
- by the reaction of fluorine with sulfur tetrafluoride SF4 in the presence of a catalyst;
- thermal decomposition of SF5CI at 200…300 °C;
- fluorination of sulfur compounds (eg COS). This method of waste-free production of SF6 gas, based on the re-fluorination of polluting products, is not yet used in the Russian Federation, like the previous two.