What is electric charge q
Electric charge, denoted in the international system of units by the letters q and Q, is considered a scalar physical quantity that determines the property of a particle or body to act as a source of an electromagnetic field and enter into direct interaction with it. In physics, there are several types of electromagnetic charged particles, and they are called positive or negative. Both units are measured in Coulombs, and can be found by calculating the product of one Ampere and one second.
Concept from the textbook
Two types of charge
Since gravitational interaction is always attraction, the masses of all bodies are non-negative. But this is not true for charges. It is convenient to describe two types of electromagnetic interaction - attraction and repulsion - by introducing two types of electric charges: positive and negative.
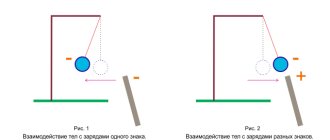
Charges of different signs attract each other, and charges of different signs repel each other. This is illustrated in Fig. 1; The balls suspended on threads are given charges of one or another sign.
Rice. 1. Interaction of two types of charges
The widespread manifestation of electromagnetic forces is explained by the fact that the atoms of any substance contain charged particles: the nucleus of an atom contains positively charged protons, and negatively charged electrons move in orbits around the nucleus.
The charges of a proton and an electron are equal in magnitude, and the number of protons in the nucleus is equal to the number of electrons in orbits, and therefore it turns out that the atom as a whole is electrically neutral. This is why, under normal conditions, we do not notice the electromagnetic influence from surrounding bodies: the total charge of each of them is zero, and charged particles are evenly distributed throughout the volume of the body. But if electrical neutrality is violated (for example, as a result of electrification), the body immediately begins to act on the surrounding charged particles.
Why there are exactly two types of electric charges, and not some other number, is currently not known. We can only assert that accepting this fact as primary provides an adequate description of electromagnetic interactions.
The charge of a proton is Cl. The charge of an electron is opposite to it in sign and is equal to Cl. Magnitude
Cl
called the elementary charge. This is the minimum possible charge: free particles with a smaller charge were not detected in experiments. Physics cannot yet explain why nature has the smallest charge and why its magnitude is exactly that.
The charge of any body always consists of an integer number of elementary charges:
If , then the body has an excess number of electrons (compared to the number of protons). If, on the contrary, the body lacks electrons: there are more protons.
Formula for finding charge
The required value can be determined from the physical and mathematical formula for current strength. In accordance with it, you need to multiply the current strength by the time it passes through the conductor. The amount of charge can be found through the formula +-ne, where n is an integer and e is equal to the value = -1.6*10^-19 Coulomb.
Note ! The charge formula is a consequence of the direct dependence of the electromagnetic field strength on the potential of its particle, which is the basic rule for finding the capacity of a charged capacitor and the amount of energy accumulated in it. In addition, the amount of charge can be calculated using the Lorentz force.
Basic formulas
Theoretical information
Electric charge is the ability of bodies to create an electromagnetic field. In physics, the section of electrostatics studies the interactions of charges that are stationary relative to the selected inertial reporting system.
What is it measured in?
The unit of measurement in the SI system is called “Coulomb” - this is an electric charge passing through a cross-section of a conductor of 1 Ampere in 1 second.
Letter designation – Q or q. Can take both positive and negative values. The name is named after the physicist Charles Coulomb, he derived a formula for finding the interaction forces between them, it is called “Coulomb’s Law”:
In it, q1, q2 are the charge modules, r is the distance between them, k is the proportionality coefficient.
The formula is similar to the law of attraction; in principle, it describes such an interaction. It has the least mass. Its electric charge is negative and it is equal to:
-1.6*10^(-19) Cl
A positron is the opposite value of an electron and also consists of one positive elementary charge.
In addition to the fact that it is discrete, quantized or measured in portions, the Law of Conservation of Charges is also valid for it, which says that in a closed system only charges of both signs can arise simultaneously. In simple terms, the algebraic (including signs) sum of the charges of particles and bodies in a closed (isolated) system always remains unchanged. It does not change with time or with the movement of the particle, it is constant during its lifetime. The simplest charged particles are conventionally compared to electric charges.
The law of conservation of electric charges was first confirmed by Michael Faraday in 1843. This is one of the fundamental laws of physics.
Conductors, semiconductors and dielectrics
There are many free charges in conductors. They move freely throughout the entire volume of the body. There are almost no free carriers in semiconductors, but if a little energy is transferred to the body, they are formed, as a result of which the body begins to conduct electric current, i.e. electric charges begin to move. Dielectrics are substances where the number of free carriers is minimal, so current cannot flow through them or can under certain conditions, for example, a very high voltage.
How to calculate using laws
Since q and Q are scalar units, they can be calculated using the laws through exact formulas derived by famous physicists. For example, in accordance with Coulomb's law, it is possible to find the magnitude and force direction of the interaction of charged particles between several stationary bodies.
You might be interested in Anti-static electricity protection products
Conservation Law
All elementary particles are divided into neutral or charged. They interact with each other within an electromagnetic field. Particles that have the same electron repel, and opposite electrons attract. In the first case, there is an excess of electrons, and in the second, a lack of electrons. Both types of particles are charged through electrification. In practice, when this phenomenon occurs, the charged particles are equal in magnitude, despite the opposite signs. When different particles attract, electrification and electron conservation occurs between them. In this case, the sum of all isolated system particles does not change, that is, q + q + q…= const.
Conservation Law
Coulomb's law
It was said above that electrically charged microparticles can be both positive and negative, and their presence is confirmed by force interaction, which O. Coulomb described with the help of experiments on scales in 1785, creating his own physical and mathematical law.
Coulomb's law is a physical law that describes the interaction of electrified particles between non-electrified ones, depending on the gap between them. According to this formulation, the more electrons a particle has, the closer it is to another unit of charge, and the force increases accordingly.
Note ! As the distance between particles increases, the strength of their interaction invariably decreases. In the mathematical formula it looks like this: F1 = F2 = K*(q1*q2/r2), where q1 and q2 are considered to be the moduli of charged microparticles, k is the proportionality coefficient, which depends on the system choice of unit, and r is the distance.
Coulomb's law
Coulomb's law
While studying the behavior of two point charges on a torsion balance, that is, those for which the distance between them significantly exceeds their dimensions, Charles Coulomb in 1785 discovered the law of interaction between electric charges. The scientist formulated this law as follows:
The magnitude of each force with which two point charges at rest interact is directly proportional to the product of their electric charges and inversely proportional to the square of the distance separating them. The interaction forces are directed along the line that connects the charged bodies.
Note that Coulomb's law does not depend on the type of charges: changing the sign of the charge will only change the direction of the acting force to the opposite, while maintaining its modulus. The proportionality coefficient in Coulomb's law depends on the dielectric constant of the medium in which the charges are considered.
Thus, the formula for the Coulomb force is written in the following form: F = k*q1*q2/r2, where q1, q2 are the magnitudes of the charges, r is the distance between the charges, k = 9*109 N*m2/Cl2 is the proportionality coefficient for vacuum.
The constant k through the universal dielectric constant ε0 and the dielectric constant of the material ε is expressed as follows: k = 1/(4*pi*ε*ε0), here pi is the number pi, and ε > 1 for any medium.
Coulomb's law is not valid in the following cases:
- when charged particles begin to move, and especially when their speeds approach near the speed of light;
- when the distance between charges is small compared to their geometric dimensions.
It is interesting to note that the mathematical form of Coulomb's law coincides with that of the law of universal gravitation, in which the role of the electric charge is played by the mass of the body.
§ 84. Electric charge and elementary particles. Law of conservation of charge
Chapter 14. ElectrostaticsFirst, let's consider the simplest case, when electrically charged bodies are at rest.
| Remember The section of electrodynamics devoted to the study of the equilibrium conditions of electrically charged bodies is called electrostatics . |
- Remember from the basic school physics course the definition of electric charge.
What charges are there?
With the words electricity, electric charge, electric current
you have met many times and managed to get used to them.
But try to answer the question: “What is an electric charge?” charge
itself is a basic, primary concept that cannot be reduced at the current level of development of our knowledge to any simpler, elementary concepts.
Let us first try to find out what is meant by the statement: “This body or particle has an electric charge.”
| Remember All bodies are built from the smallest particles, which are indivisible into simpler ones and therefore are called elementary . |
Elementary particles have mass and due to this they are attracted to each other according to the law of universal gravitation. As the distance between particles increases, the gravitational force decreases in inverse proportion to the square of this distance. Most elementary particles, although not all, also have the ability to interact with each other with a force that also decreases in inverse proportion to the square of the distance, but this force is many times greater than the force of gravity.
Thus, in the hydrogen atom, shown schematically in Figure 14.1, the electron is attracted to the nucleus (proton) with a force 1039 times greater than the force of gravitational attraction.
| Important If particles interact with each other with forces that decrease with increasing distance in the same way as the forces of universal gravity, but exceed the gravitational forces many times, then these particles are said to have an electric charge. The particles themselves are called charged . |
There are particles without an electric charge, but there is no electric charge without a particle.
| Remember The interaction of charged particles is called electromagnetic . |
Electric charge determines the intensity of electromagnetic interactions, just as mass determines the intensity of gravitational interactions.
The electric charge of an elementary particle is not a special mechanism in the particle that could be removed from it, decomposed into its component parts and reassembled. The presence of an electric charge on an electron and other particles only means the existence of certain force interactions between them.
| We, in essence, know nothing about charge if we do not know the laws of these interactions. Knowledge of the laws of interactions should be included in our ideas about charge. These laws are not simple, and it is impossible to outline them in a few words. Therefore, it is impossible to give a sufficiently satisfactory brief definition of the concept electric charge . |
Two signs of electric charges. All bodies have mass and therefore attract each other. Charged bodies can both attract and repel each other. This most important fact, familiar to you, means that
| It is important that in nature there are particles with electric charges of opposite signs; in the case of charges of the same sign, the particles repel, and in the case of different signs, they attract. |
| Read on the Internet or other sources about the history of the discovery of the electron. |
Charge of elementary particles - protons
, which are part of all atomic nuclei, is called positive, and the charge of
electrons
is called negative. There are no internal differences between positive and negative charges. If the signs of the particle charges were reversed, then the nature of electromagnetic interactions would not change at all.
Elementary charge. In addition to electrons and protons, there are several other types of charged elementary particles. But only electrons and protons can exist in a free state indefinitely. The rest of the charged particles live less than a millionth of a second. They are born during collisions of fast elementary particles and, having existed for an insignificantly short time, decay, turning into other particles. You will become familiar with these particles in 11th grade.
Neutrons are particles that have no electrical charge.
. Its mass is only slightly greater than the mass of a proton. Neutrons, together with protons, are part of the atomic nucleus. If an elementary particle has a charge, then its value is strictly defined.
Charged bodies. Electromagnetic forces in nature play a huge role due to the fact that all bodies contain electrically charged particles. The constituent parts of atoms—nuclei and electrons—have an electrical charge.
| Important The effect of electromagnetic forces between bodies is not directly detected, since the bodies in their normal state are electrically neutral. |
An atom of any substance is neutral because the number of electrons in it is equal to the number of protons in the nucleus. Positively and negatively charged particles are connected to each other by electrical forces and form neutral systems.
A macroscopic body is electrically charged if it contains an excess amount of elementary particles with any one sign of charge. Thus, the negative charge of a body is due to the excess number of electrons compared to the number of protons, and the positive charge is due to the lack of electrons.
| Important In order to obtain an electrically charged macroscopic body, that is, to electrify it, it is necessary to separate part of the negative charge from the positive charge associated with it or transfer a negative charge to a neutral body. |
This can be done using friction. If you run a comb through dry hair, a small part of the most mobile charged particles - electrons - will move from the hair to the comb and charge it negatively, and the hair will charge positively.
| Electrify the ruler by rubbing it with a cloth. Cut small pieces of paper and examine how the ruler works on them. After a while, bring the ruler to the pieces of paper again. Is the line still charged? Explain your observations. |
End of paragraph >>>