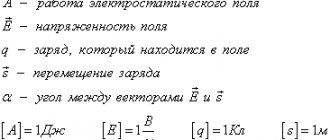What are conductors and dielectrics
Conductors are substances that have in their structure a mass of free electrical charges that can move under the influence of external force throughout the entire volume of the material.
The group of conductors in an electrostatic field includes metals and their compounds, some types of electrical coal, solutions of salts (acids, alkalis), and ionized gases.
The best conductive material is considered to be metal, for example gold, platinum, copper, aluminum. Non-metallic substances that conduct current include carbon.
Conductor
Dielectrics are substances whose properties are opposite to conductors. In the absence of heating, charged particles in a neutral atom are closely interconnected and cannot move within the bulk of the material. In this regard, electric current cannot flow in a non-conductor.
Dielectric
Materials that do not conduct electric current include: ceramics, rubber, paper, glass, porcelain, resin, dry wood. Gas is considered the best dielectric. The qualities of dielectrics depend on the temperature and humidity of the environment in which they are located.
Important! As humidity increases, dielectrics may lose their non-conducting properties.
Conductors and dielectrics are actively used in the electrical field. Example - the material from which wires (cables) are made are conductors made of metal. Insulating shells for them are made from dielectrics - polymers.
Properties of materials
The best conductors are those made from silver, gold or platinum. Their widespread use is limited only by the high cost of the material. Such products have found application in the defense and space industries. In these areas, it is important to ensure the highest quality equipment, regardless of cost.
The scope of application of copper and aluminum materials is much wider. Low cost and excellent conductive qualities have made it possible to use them in many industries.
In dielectrics, an increase in temperature can lead to the appearance of free electrical charges. These are electrons that have been detached from the nucleus due to temperature fluctuations. Usually this is a small amount of free charges. But there are insulators in which this number reaches significant sizes. In this case, the insulating qualities of the dielectric deteriorate.
Note! A dielectric is considered reliable if the small leakage current that occurs in it does not interfere with the operation of the entire system.
The best dielectric is absolute vacuum, as well as completely purified water. But they cannot be found in nature, and it is very difficult to create them artificially. The inclusion of any impurity in a liquid provides it with conducting qualities.
Conductors and dielectrics in an electrostatic field
As you know from your eighth grade physics course, all bodies can be classified according to their ability to conduct electric current. The body can be a conductor, semiconductor or dielectric. Conductors are bodies that conduct electricity, while dielectrics are bodies that do not conduct electricity.
Semiconductors are bodies that change their conductivity properties depending on external conditions.
But we'll talk about semiconductors later, and today we'll look at conductors and dielectrics.
Let's consider what happens to a conductor placed in an electrostatic field. Of course, conductors primarily include metals in which so-called free charges exist. Free charges are electrical charges that can move inside a conductor.
As you know, metals exhibit metallic bonding. Neutral metal atoms begin to interact with each other, as a result of which some electrons break away from the atoms and become free. These electrons begin to participate in thermal motion and can move throughout the conductor in random directions. In other words, free electrons in a conductor behave like gas molecules. Since all atoms are initially electrically neutral, if they lose an electron they become positively charged ions.
Thus, the following picture is observed in conductors: positively charged ions are surrounded by the so-called electron gas. Of course, one should not think that electrons form some kind of real gas. It’s just that their movement is very reminiscent of the chaotic movement of gas molecules.
Let's consider the case when a metal conductor is in a uniform electrostatic field.
As you know, under the influence of an electric field, free electrons come into ordered motion (that is, an electric current arises in the conductor). As a result, one side of the conductor becomes negatively charged and the other positively charged. This phenomenon is called electrostatic induction. That is, electrostatic induction is the phenomenon of inducing one’s own electrostatic field under the influence of an external electric field.
So, due to electrostatic induction, another electrostatic field arises, created by the emerging charges. According to the principle of field superposition, this field is superimposed on the external field and compensates for it. From this we can draw a very important conclusion: the electrostatic field strength inside the conductor is zero:
This fact is used to create electrostatic protection: devices sensitive to the electric field are placed in metal boxes. Nowadays, even some types of workwear include modern electrically conductive materials that create a closed space inside the suit, protected from the effects of electric fields.
For the first time, an experiment confirming the absence of an electrostatic field inside a conductor was carried out by Michael Faraday back in 1836. At his direction, a large wooden cage was covered with sheets of tin foil (which is a conductor). The cell was first isolated from the ground and heavily charged (so that when bodies approached it, sparks flew from its surface).
However, Faraday himself was completely calm inside this cage. Moreover, in his hands there was a working electroscope, which showed the complete absence of an electric field. Subsequently, such structures were called “Faraday cages”.
One more important fact should be noted: near the surface (outside the conductor), the electrostatic field strength lines are perpendicular to this surface.
If this were not so, and some line of tension was not perpendicular to the surface, then this would lead to the movement of free charges. This movement continues until all lines of force become perpendicular to the surface of the conductor. It must be said that the entire static charge of any conductor is on the surface of this conductor. This is easy to verify, since we have already found out that the electrostatic field strength inside the conductor is zero. Consequently, there is no charge inside the conductor, since otherwise it would create a non-zero voltage.
Now let's talk about dielectrics. Dielectrics behave differently in an electrostatic field than conductors. Dielectrics, on the other hand, do not conduct current, but an electric field can exist within them.
The fact is that free charges do not arise in dielectrics, since there is a fairly strong connection between the nuclei of atoms and electrons. Let us give two classic examples of electric charge distribution. As you know, the hydrogen nucleus consists of one proton, and one electron revolves around this proton. In general, the atom is electrically neutral. An electron rotates around a proton at a very high speed: in one second it makes about 1015 revolutions. This tells us that every microsecond an electron finds itself at any point in its orbit millions of times. Therefore, we can safely assume that, on average over time, the center of the negative charge distribution is located in the center of the atom, that is, it coincides with the positively charged nucleus.
However, there are other cases. For example, a molecule of table salt consists of a sodium atom and a chlorine atom. From your chemistry course, you know that a chlorine atom has 7 valence electrons, while a sodium atom has only one valence electron. Therefore, during the formation of the molecule, the chlorine atom captures an electron from sodium, resulting in the formation of a system of two ions. Now the center of the negative charge distribution is on the chlorine ion, and the center of the positive charge distribution is on the sodium ion. However, overall the molecule remains electrically neutral. Such systems are called electric dipoles.
Electric dipole
In this regard, there are two types of dielectrics: non-polar and polar. Non-polar dielectrics are dielectrics consisting of atoms or molecules in which the centers of distribution of positive and negative charges coincide.
Conversely, polar dielectrics are dielectrics consisting of atoms or molecules in which the centers of distribution of positive and negative charges do not coincide.
We will talk about the polarization of dielectrics in more detail in one of the following lessons. Now let's look at a quantity characterizing the property of a dielectric medium, which is called dielectric constant
.
This value shows how many times the Coulomb force of interaction between two point charges in a given medium is less than the Coulomb force of interaction of the same charges in a vacuum:
Thus, we can write Coulomb’s law for an arbitrary medium:
The dielectric constant is added to the formula, that is, a characteristic of the medium. The dielectric constants of many media have been measured and tabulated. These quantities are measured experimentally, for example, by measuring the Coulomb forces of the same charges in different media.
Properties of conductors
Resistivity
The main characteristics of electrical conductors are:
- resistance,
- electrical conductivity.
When electrons move through a conducting substance, they collide with ions and atoms. This leads to resistance.
If a potential difference is created between two conductors, then an electric current will flow through the third one connecting them. The direction of its movement will be from greater potential to less. In this case, the carriers will be electrons that are not connected to each other, which determine the value of the electrical conductivity of the substance.
Electrical conductivity is the ability of a material to pass electric current. This indicator is inversely proportional to the resistance of the material, measured in siemens, see.
Depending on the charge carriers, electrical conductivity can be:
- electronic,
- ionic,
- hole
Conductor with electronic conductivity
Note! A reliable conductor is characterized by low resistance to the flow of moving electrons and, accordingly, high electrical conductivity. The greatest conductivity is the property of the best conductor.
The selection of conductive materials should be carried out in accordance with their properties:
- Electrical (resistivity and temperature coefficient of resistance);
- Physical (melting degree, density);
- Mechanical (resistance to stretching, bending, machine processing);
- Chemical (interaction with the environment, the possibility of connection during welding, soldering).
Metals without impurities have low resistivity. For alloys this figure increases. Resistance also increases with increasing temperature.
Important! When cooled to critical values, the resistance of most conductive substances drops to zero. This property is called superconductivity.
When choosing conductors for electrical installations, power lines, protective grounding and other applications, it is important to take into account all the qualities of the materials.
Conductors and dielectrics
All existing natural substances, according to the degree of electrical conductivity, are conventionally divided into three groups: conductors of electric current, dielectric and semiconductor materials.
Separation of materials by electrical conductivity
What are conductors and dielectrics
Conductors are substances that have in their structure a mass of free electrical charges that can move under the influence of external force throughout the entire volume of the material.
The group of conductors in an electrostatic field includes metals and their compounds, some types of electrical coal, solutions of salts (acids, alkalis), and ionized gases.
The best conductive material is considered to be metal, for example gold, platinum, copper, aluminum. Non-metallic substances that conduct current include carbon.
Dielectrics are substances whose properties are opposite to conductors. In the absence of heating, charged particles in a neutral atom are closely interconnected and cannot move within the bulk of the material. In this regard, electric current cannot flow in a non-conductor.
Materials that do not conduct electric current include: ceramics, rubber, paper, glass, porcelain, resin, dry wood. Gas is considered the best dielectric. The qualities of dielectrics depend on the temperature and humidity of the environment in which they are located.
Important! As humidity increases, dielectrics may lose their non-conducting properties.
Conductors and dielectrics are actively used in the electrical field. Example - the material from which wires (cables) are made are conductors made of metal. Insulating shells for them are made from dielectrics - polymers.
The best conductors are those made from silver, gold or platinum. Their widespread use is limited only by the high cost of the material. Such products have found application in the defense and space industries. In these areas, it is important to ensure the highest quality equipment, regardless of cost.
The scope of application of copper and aluminum materials is much wider. Low cost and excellent conductive qualities have made it possible to use them in many industries.
In dielectrics, an increase in temperature can lead to the appearance of free electrical charges. These are electrons that have been detached from the nucleus due to temperature fluctuations. Usually this is a small amount of free charges. But there are insulators in which this number reaches significant sizes. In this case, the insulating qualities of the dielectric deteriorate.
Note! A dielectric is considered reliable if the small leakage current that occurs in it does not interfere with the operation of the entire system.
The best dielectric is absolute vacuum, as well as completely purified water. But they cannot be found in nature, and it is very difficult to create them artificially. The inclusion of any impurity in a liquid provides it with conducting qualities.
Properties of conductors
The main characteristics of electrical conductors are:
- resistance,
- electrical conductivity.
When electrons move through a conducting substance, they collide with ions and atoms. This leads to resistance.
If a potential difference is created between two conductors, then an electric current will flow through the third one connecting them. The direction of its movement will be from greater potential to less. In this case, the carriers will be electrons that are not connected to each other, which determine the value of the electrical conductivity of the substance.
Electrical conductivity is the ability of a material to pass electric current. This indicator is inversely proportional to the resistance of the material, measured in siemens, see.
Depending on the charge carriers, electrical conductivity can be:
- electronic,
- ionic,
- hole
Conductor with electronic conductivity
Note! A reliable conductor is characterized by low resistance to the flow of moving electrons and, accordingly, high electrical conductivity. The greatest conductivity is the property of the best conductor.
The selection of conductive materials should be carried out in accordance with their properties:
- Electrical (resistivity and temperature coefficient of resistance);
- Physical (melting degree, density);
- Mechanical (resistance to stretching, bending, machine processing);
- Chemical (interaction with the environment, the possibility of connection during welding, soldering).
Metals without impurities have low resistivity. For alloys this figure increases. Resistance also increases with increasing temperature.
Important! When cooled to critical values, the resistance of most conductive substances drops to zero. This property is called superconductivity.
When choosing conductors for electrical installations, power lines, protective grounding and other applications, it is important to take into account all the qualities of the materials.
Dependence of conductor resistance on current frequency
When exposed to electric current, the induction of a magnetic field occurs inside a straight conductor and in the space surrounding it. Magnetic lines form concentric circles.
Distribution of alternating current across a cross section What is electrical resistance
If a current-carrying conductor is conditionally divided into several current filaments parallel to each other, then it can be established that the closer the current filament is to the axis of the conductor, the greater the magnetic flux that closes inside it covers it. The inductance of the filament and the inductive reactance are proportional to the magnetic flux associated with it.
In this regard, in threads with alternating current located inside the conductive substance, greater inductive reactance occurs than in threads located outside. An uneven current across the cross-section is formed, increasing from the axis to the surface of the conductor, which explains the increase in the resistance of the conductors to alternating current. This phenomenon is called the skin effect.
Due to the uneven distribution of current density, the resistance of the conductor increases. At a low frequency of 50 Hz and a small cross-section of copper wire, the phenomenon of surface effect is almost imperceptible. With a significant increase in the frequency and cross-section of the iron conductor, this phenomenon will be more active.
Note! The higher the frequency of the current in the circuit, the closer to the surface of the conductor the electrical charges are, and the more its resistance increases.
Formula for determining conductor length
Current resistance: formula
You can find the length of the conductor by directly measuring it, for example, with a tape measure.
If you need to calculate the length of hidden electrical wiring in a home, you need to take into account that it is usually laid horizontally along the walls at a distance of 15-20 cm from the ceiling. Vertically, at right angles, make drops on switches and sockets.
If the conductor is difficult to reach (grounding conductors) or is long, this method may be difficult to implement.
Then the length of the conductor is determined in a different way. To do this you need to prepare:
- construction tape,
- tester,
- calipers,
- table of electrical conductivity of metals.
First you need to measure the resistance of individual sections of electrical wiring. Next, determine the cross-section of the wire and the material from which it is made. Typically, aluminum or copper conductive materials are used in everyday life.
From the formula for determining resistance ( R = r * L * s), find the length of the conductor using the formula:
L = R / r*s,
Where:
- L – wire length,
- R is its resistance,
- r – resistivity of the material (for copper it ranges from 0.0154 to 0.0174 Ohm, for aluminum – from 0.0262 to 0.0278 Ohm),
- s – cross-sectional area of the wire.
Calculate the wire cross-section:
S = π/4 * D2,
Where:
- π – a number approximately equal to 3.14;
- D is the diameter measured with a caliper.
If you need to find the length of a wire wound into a coil, determine the length of one turn in meters and multiply by the number of turns.
If the coil is round, measure its diameter, multiply by the number π and the number of turns:
L = d * π * n,
Where:
- d – coil diameter,
- n – number of turns of wire.
Types of conductors
The state of electrically conductive materials can be solid, liquid, or gaseous.
Solids are groups of metals, their alloys and some modifications of carbon. Metals conduct heat and electricity well.
Liquids are molten metals and electrolytes. At low temperatures, the liquid conductor can be mercury or gallium. The melting point of the remaining elements is too high.
The flow of current through a metal, whether in a solid or liquid state, occurs through the movement of free electrons. Due to this, its electrical conductivity is called electronic, and the substance itself is called a conductor of the first kind.
A conductor of the second type (electrolyte) is an acidic, alkaline, saline solution and a melt of ionic compounds. Molecules (ions) are transported in it simultaneously with the movement of current, so over time the structure of the electrolyte changes, and the electrolysis product is deposited on the electrodes.
In a low-intensity electric field, any gas or vapor does not conduct current.
But if the voltage reaches its maximum critical point, when impact and photo-ionization begin, the gas can become a conductor with electronic and ionic conductivity.
When there are equal numbers of electrons and positive ions per unit volume, the highly ionized gas will become a balanced, electrically conductive substance called plasma.
Properties of dielectrics
The choice of dielectrics should be made in accordance with their properties:
- Electrical: breakdown voltage (at which breakdown occurs), electrical strength (field strength at which breakdown occurs);
- Physico-chemical: resistance to heat (the ability to withstand operating temperatures for a long time), cold resistance (the ability to withstand temperature changes), wettability (the ability to reject moisture);
- Chemical: resistance to aggressive environments, solubility in varnishes, possibility of gluing;
- Mechanical: radiation resistance, viscosity (for liquid dielectrics), corrosion protection, tensile strength, possibility of tooling.
What is a semiconductor
By designation, a semiconductor is a substance whose electrical conductivity is less than that of a metal and greater than that of a dielectric.
The difference between a semiconductor is that its electrical conductivity depends on the temperature regime and the volume of impurities in the composition. The material has both conductive and dielectric characteristics.
As the temperature increases, the electrical conductivity of a substance increases, and the resistance level decreases. As the temperature decreases, the resistance tends to infinity.
Note! When the temperature reaches zero, the semiconductor behaves as an insulator.
Due to their unique properties, semiconductors are used in many industries: low-power SMD on printed circuit boards, and high-power devices, for example, thyristors in power converter technology.
Zone theory
The band theory of solids is the theory of the movement of valence electrons in the potential field of a crystal lattice. Quantum mechanics believes that free electrons can have any energy, the spectrum of which is continuous.
The electrons of isolated atoms have a certain discrete amount of energy. When individual atoms combine into molecules and form a substance, the electronic levels of the atom shift. Thus, from the energy levels of individual atoms in a solid, bands of energy level zones are formed.
The upper filled band, the valence band, corresponds to the energy level of the valence electrons of the outer shell. The closest to it, unfilled, is the conduction band. The relative position of both zones determines the processes occurring in a solid, and materials are classified into groups: conductors, semiconductors, dielectrics.
In conductors, the conduction band and valence band are combined. The resulting overlap zone allows the electron to move freely when receiving even small amounts of energy.
In semiconductors, the bands do not overlap. The distance between them, called the band gap, is less than 2.0 eV. At zero temperature, there are no electrons in the conduction band, and the valence band is filled with them. As the temperature increases, some electrons are thrown into the conduction band due to thermal motion. The semiconductor becomes electrically conductive.
In dielectrics, the zones, just like in semiconductors, do not overlap. The band gap here is more than 2.0 eV. In order to transfer electrons from the valence zone to the conduction zone, it is necessary to significantly increase the temperature. At low degrees, no electric current is conducted.
Superconductivity
The property of a material to have zero electrical resistance at a temperature below a certain value is called superconductivity.
For some conductive substances, this ability occurs at cold temperatures close to the chemical state of liquid helium.
In 1986, substances with high-temperature superconductivity were discovered. For example, ceramics made of oxygen, barium, copper, lanthanum do not conduct current under normal conditions, but due to heating they become a superconductor.
In practice, substances are used that transmit electric current at 58 degrees Kelvin or more, that is, at a temperature above the boiling point of nitrogen.
Solid high-temperature superconductors are most often used. Liquid and gaseous ones are used less frequently. All these materials are necessary for the manufacture of modern electrical devices of various capacities.
Source: https://amperof.ru/teoriya/provodniki-i-dielektriki.html
Dependence of conductor resistance on current frequency
When exposed to electric current, the induction of a magnetic field occurs inside a straight conductor and in the space surrounding it. Magnetic lines form concentric circles.
Alternating current distribution across the cross section
How to calculate amperes
If a current-carrying conductor is conditionally divided into several current filaments parallel to each other, then it can be established that the closer the current filament is to the axis of the conductor, the greater the magnetic flux that closes inside it covers it. The inductance of the filament and the inductive reactance are proportional to the magnetic flux associated with it.
In this regard, in threads with alternating current located inside the conductive substance, greater inductive reactance occurs than in threads located outside. An uneven current across the cross-section is formed, increasing from the axis to the surface of the conductor, which explains the increase in the resistance of the conductors to alternating current. This phenomenon is called the skin effect.
Due to the uneven distribution of current density, the resistance of the conductor increases. At a low frequency of 50 Hz and a small cross-section of copper wire, the phenomenon of surface effect is almost imperceptible. With a significant increase in the frequency and cross-section of the iron conductor, this phenomenon will be more active.
Note! The higher the frequency of the current in the circuit, the closer to the surface of the conductor the electrical charges are, and the more its resistance increases.
What is a conductor, semiconductor and dielectric according to Band Theory
In the electrical power industry, three main groups of materials can be distinguished: conductor, semiconductor and dielectric. Their main difference is that they have different electrical conductivity. In this article we will talk about the differences between such materials and their behavior in an electric field.
What is a conductor
So, a conductor is a material (substance, medium) that perfectly conducts electric current. The so-called free charged particles (electrons or ions) present in the substance are able to move freely throughout the entire volume of the substance, and when an electrical voltage is applied, they create a conduction current.
The main characteristic of a conductor is its “resistance” ( R
), measured in Ohms or the inverse quantity called “conductivity”, is found by the formula:
G = 1/R
And this value is measured in Siemens
.
Conductors include: most metals, carbon (coal or graphite), various solutions of salts and acids.
Conductors in which charge transfer is carried out primarily due to the movement of electrons (electron emission) are called conductors of the first kind. If charge movement in conductors is carried out by ions (electrolytes), then they are called second-order conductors.
Metals are most widely used because they have the best conductivity, which means they have less resistance to the flow of electric current.
For example, the cores of all supply wires (cords) are made of metals that are conductors.
What is a dielectric
Dielectrics are those substances that have high resistance and do not transmit electric current or conduct it in small quantities.
This is due to the fact that in such materials there are extremely few free charge carriers due to the rather strong atomic bond. Therefore, when exposed to an electric field, there is simply no current in the dielectric.
Dielectrics include materials such as: glass, porcelain, ceramics, textolite, carbolite, distilled water (without salt impurities), dry wood, rubber, etc.
Dielectrics are also extremely widely used in everyday life. Wire insulation and electrical appliance housings are made of dielectric materials.yandex.ru
But if you create certain conditions, for example, greatly increase the operating voltage, then the dielectric can become a conductor. Surely you have heard the expression “insulation breakdown”.
The main characteristic of any dielectric is the electrical strength (this value is equal to the breakdown voltage).
What is a semiconductor
As can be seen even from the name itself, semiconductors occupy an intermediate position between conductors and dielectrics. Semiconductors in their original state do not transmit electric current, but as soon as energy is applied to the semiconductor material, the semiconductor turns from a dielectric into a conductor.
Similar elements are used in radio electronics; they are used to produce transistors, thyristors, diodes, LEDs, etc.
The division of substances into conductors, semiconductors and dielectrics is explained using the Band Theory of Solids
. She, of course, is not easily accepted by everyone, but it is highly desirable to get to know her.
Band theory of solids
So, the difference between dielectrics, conductors and semiconductors can be explained by band theory. It sounds like this:
As is known from the Bohr atom model, electrons in an atom are located in certain orbits
yandex.ru
In the crystal lattice of a solid, the orbits of electrons change under the inevitable influence of neighboring atoms and electrons. And for this reason, there is a shift in the energy levels of electron confinement.
From orbits close to the nucleus of an atom, electrons can move to another level purely theoretically, but from external orbits, which in a solid body are blurred into sublevels, the transition of electrons between them can be carried out quite easily.
And when an electric potential is applied, electrons randomly jumping around the outer orbits of neighboring atoms acquire a single vector of motion and we observe an electric current.
Therefore, the lower layer, where there are freely moving electrons, is called the conduction band.
The valence band is the region of allowed energies and is located below the conduction band.
In order for an electron to move from the valence band to the conduction band, it must cross the so-called band gap.
It is expressed numerically in electron volts. And the energy levels of semiconductors, conductors and dielectrics can be schematically represented as follows:
As can be seen from the figure above, the conductor has no band gap, that is, the valence band and conduction band have an overlap region. This means that in such a material, even with a slight application of energy, electrons begin to actively move within the conductor body.
A semiconductor has a band gap between levels. Its width shows how much energy needs to be applied to the semiconductor for electrons to begin moving, that is, current begins to flow.
And in a dielectric, the forbidden region is so wide that the transition of electrons from the valence region to the conductive region is practically excluded. Since significant energy will be required to overcome this barrier, which will cause the destruction of the dielectric.
Conclusion
That's all I wanted to tell you about dielectrics, conductors and semiconductors. If you found the article interesting and useful, please rate it. And thanks for your attention!
Source: https://zen.yandex.ru/media/id/5aef12c13dceb76be76f1bb1/5c11422a220e2000ab355e80
Formula for determining conductor length
Ampere - what is it?
You can find the length of the conductor by directly measuring it, for example, with a tape measure. If you need to calculate the length of hidden electrical wiring in a home, you need to take into account that it is usually laid horizontally along the walls at a distance of 15-20 cm from the ceiling. Vertically, at right angles, make drops on switches and sockets. If the conductor is difficult to reach (grounding conductors) or is long, this method may be difficult to implement.
Then the length of the conductor is determined in a different way. To do this you need to prepare:
- construction tape,
- tester,
- calipers,
- table of electrical conductivity of metals.
First you need to measure the resistance of individual sections of electrical wiring. Next, determine the cross-section of the wire and the material from which it is made. Typically, aluminum or copper conductive materials are used in everyday life.
From the formula for determining resistance ( R = r * L * s), find the length of the conductor using the formula:
L = R / r*s,
Where:
- L – wire length,
- R is its resistance,
- r – resistivity of the material (for copper it ranges from 0.0154 to 0.0174 Ohm, for aluminum – from 0.0262 to 0.0278 Ohm),
- s – cross-sectional area of the wire.
Calculate the wire cross-section:
S = π/4 * D2,
Where:
- π – a number approximately equal to 3.14;
- D is the diameter measured with a caliper.
If you need to find the length of a wire wound into a coil, determine the length of one turn in meters and multiply by the number of turns.
If the coil is round, measure its diameter, multiply by the number π and the number of turns:
L = d * π * n,
Where:
- d – coil diameter,
- n – number of turns of wire.
Types of conductors
The state of electrically conductive materials can be solid, liquid, or gaseous.
Solids are groups of metals, their alloys and some modifications of carbon. Metals conduct heat and electricity well.
Liquids are molten metals and electrolytes. At low temperatures, the liquid conductor can be mercury or gallium. The melting point of the remaining elements is too high.
The flow of current through a metal, whether in a solid or liquid state, occurs through the movement of free electrons. Due to this, its electrical conductivity is called electronic, and the substance itself is called a conductor of the first kind.
A conductor of the second type (electrolyte) is an acidic, alkaline, saline solution and a melt of ionic compounds. Molecules (ions) are transported in it simultaneously with the movement of current, so over time the structure of the electrolyte changes, and the electrolysis product is deposited on the electrodes.
In a low-intensity electric field, any gas or vapor does not conduct current. But if the voltage reaches its maximum critical point, when impact and photo-ionization begin, the gas can become a conductor with electronic and ionic conductivity. When there are equal numbers of electrons and positive ions per unit volume, the highly ionized gas will become a balanced, electrically conductive substance called plasma.
Dielectrics in an electric field
TOE › Electric field
Dielectric
(insulator) - a substance, medium, material that practically does not conduct electric current.
The main property of a dielectric
is its ability to polarize in an external electric field.
The concentration of free charge carriers in the dielectric
does not exceed 108 cm−3.
Let us consider in more detail the processes in a dielectric placed in an external electric field, for example, between oppositely charged electrodes.
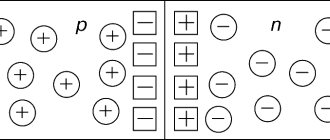
In one group of dielectrics, called non-polar , in the absence of an external (main) field, the positively and negatively charged particles included in the molecules (atoms) seem to balance each other (there is no own field); their molecules are electrically neutral or nonpolar (Fig. 1, a). In such dielectrics, under the influence of an external field, the electrical center of negative charges (electrons) shifts towards the direction of the field (Fig. 1, b). From the point of view of electrical properties, such a molecule in an external field can be considered as a dipole, i.e. a pair of opposite point charges +q and -q (Fig. 1c), located at a small distance l from each other (dipole arm). The charges forming dielectric dipoles are called bound, and the product of charge q and arm l is called electric moment of the dipole :
p = ql
The electric torque is considered as a vector quantity p directed from the negative charge of the dipole to the positive one.
Figure 1 - non-polar molecule a) in the absence of an external field; b) in the presence of an external field; c) its equivalent dipole
Figure 2 - Polarized dielectric
Thus, nonpolar molecules in an external field become dipoles, the electric moments p of which tend to be located in the direction of the external field, and the dielectric is polarized (Fig. 2). When the external field disappears, the displacement disappears and the molecules become electrically neutral again. The considered polarization is called deformation.
In each group of dielectrics, called polar , the molecules are always polar (the electrical centers of the electrons and molecules are located asymmetrically relative to the nuclei). A polar molecule can be considered a dipole with charges +q and -q and momentum p = ql . In the absence of an external field, all dipoles are located chaotically (Fig. 3, a) and the total electric moment of the dielectric is zero. When an external field appears, its forces tend to orient the dipoles in the direction of the field. As a result, the dipoles will rotate somewhat in the direction of the field and the dielectric acquires an electric moment (Fig. 3.6). This polarization is called orientational.
With one or another polarization of the dielectric, the field of its dipoles, or the polarization field Ep (Fig. 4.12), is directed
Figure 3 - Polar molecules
from positive charges to negative ones, i.e. opposite to the external field Evn. The resulting field strength E, equal to the algebraic sum of the external field and polarization field strengths, is less than the external field strength, i.e.
E = Ein - Ep
The stronger the dielectric is polarized, the weaker the resulting field, i.e., the lower its intensity E at the same external field, and therefore, the greater its dielectric constant Er.
For a dielectric located in a periodically changing external electric field, the displacement of charges will also be periodic, which causes heating of the dielectric . The higher the frequency the external field changes, the stronger the heating of the dielectric. This phenomenon is used to heat and dry wet materials to produce or accelerate chemical reactions that require elevated temperatures.
The power used to heat the dielectric during periodic displacement of dielectric charges (bound charges) and per unit volume is called specific dielectric losses.
Properties of dielectrics
The choice of dielectrics should be made in accordance with their properties:
- Electrical: breakdown voltage (at which breakdown occurs), electrical strength (field strength at which breakdown occurs);
- Physico-chemical: resistance to heat (the ability to withstand operating temperatures for a long time), cold resistance (the ability to withstand temperature changes), wettability (the ability to reject moisture);
- Chemical: resistance to aggressive environments, solubility in varnishes, possibility of gluing;
- Mechanical: radiation resistance, viscosity (for liquid dielectrics), corrosion protection, tensile strength, possibility of tooling.
What is a semiconductor
By designation, a semiconductor is a substance whose electrical conductivity is less than that of a metal and greater than that of a dielectric.
Semiconductors
The difference between a semiconductor is that its electrical conductivity depends on the temperature regime and the volume of impurities in the composition. The material has both conductive and dielectric characteristics.
As the temperature increases, the electrical conductivity of a substance increases, and the resistance level decreases. As the temperature decreases, the resistance tends to infinity.
Note! When the temperature reaches zero, the semiconductor behaves as an insulator.
Due to their unique properties, semiconductors are used in many industries: low-power SMD on printed circuit boards, and high-power devices, for example, thyristors in power converter technology.
Table: what is the difference between conductors and dielectrics?
| Conductor | Dielectric | |
| Availability of free electrons | Present in large numbers | Absent, or present, but very little |
| The ability of materials to conduct electric current | Has a good time | Does not conduct, or the current is negligible |
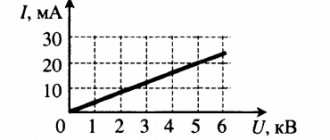
| What happens when the applied voltage increases | The current passing through a conductor increases according to Ohm's law | The current passing through the dielectric changes slightly and, upon reaching a certain value, electrical breakdown occurs |
| Materials | Gold, silver, copper and its alloys, aluminum and alloys, iron and others | Ebonite, fluoroplastic, rubber, mica, various plastics, polyethylene and other materials |
| Resistance | from 10-5 to 10-8 degrees Ohm/m | 1010 – 1016 Ohm/m |
| The influence of foreign impurities on the resistance of the material | Impurities impair the conductivity properties of the material, which impairs its properties | Impurities improve the conductivity of the material, which worsens its properties |
| Changes in properties with changes in ambient temperature | As the temperature increases, the resistance increases, and as the temperature decreases, it decreases. At very low temperatures - superconductivity. | As the temperature increases, the resistance decreases. |
Zone theory
The band theory of solids is the theory of the movement of valence electrons in the potential field of a crystal lattice. Quantum mechanics believes that free electrons can have any energy, the spectrum of which is continuous.
The electrons of isolated atoms have a certain discrete amount of energy. When individual atoms combine into molecules and form a substance, the electronic levels of the atom shift. Thus, from the energy levels of individual atoms in a solid, bands of energy level zones are formed.
The upper filled band, the valence band, corresponds to the energy level of the valence electrons of the outer shell. The closest to it, unfilled, is the conduction band. The relative position of both zones determines the processes occurring in a solid, and materials are classified into groups: conductors, semiconductors, dielectrics.
Zone classification
In conductors, the conduction band and valence band are combined. The resulting overlap zone allows the electron to move freely when receiving even small amounts of energy.
In semiconductors, the bands do not overlap. The distance between them, called the band gap, is less than 2.0 eV. At zero temperature, there are no electrons in the conduction band, and the valence band is filled with them. As the temperature increases, some electrons are thrown into the conduction band due to thermal motion. The semiconductor becomes electrically conductive.
In dielectrics, the zones, just like in semiconductors, do not overlap. The band gap here is more than 2.0 eV. In order to transfer electrons from the valence zone to the conduction zone, it is necessary to significantly increase the temperature. At low degrees, no electric current is conducted.
Dielectrics in an electric field. Polar and non-polar non-polar dielectrics.
Dielectrics in an electric field do not behave like conductors, although they have something in common. Dielectrics differ from conductors in that they do not contain free charge carriers. Still, they are there, but in very small quantities. In conductors, such charge carriers are electrons that move freely along the crystal lattice of metals. But in dielectrics, electrons are tightly bound to their atoms and cannot move freely. When a dielectric is introduced into an electric field, electrification occurs in it just like in a conductor. The difference between dielectrics is that electrons cannot move freely throughout the volume as happens in conductors. But under the influence of an external electric field, a certain charge displacement appears inside the molecule of the dielectric substance. The positive one moves along the direction of the field, and the negative one moves against it. As a result, the surface receives a certain charge. The process of charge formation on the surface of dielectrics under the influence of an electric field is called polarization of the dielectric. All dielectrics are divided into two categories. Dielectrics belonging to the first category have molecules that form dipoles even in the absence of an external electric field. They are called polar. Polar dielectrics include water, ammonia, acetone and ether. The dipoles of such dielectrics in the absence of a field are located chaotically due to thermal motion. And, therefore, the charge on the surface of such a substance is zero. But when it is introduced into an external electric field, the dipoles, that is, the molecule, tend to turn along the field. It turns out that the positive charge of the previous dipole looks at the negative charge of the next one. Therefore, they compensate each other. But dipoles located near the surface itself do not have a pair. Thus, uncompensated bound charges are formed on the surface of the material. On the one hand there are positive and on the other there are negative. But this is prevented by the thermal movement of molecules.Figure 1 - polarization of a polar dielectric
The second category of dielectrics are those that have positive and negative charges inside the molecule in a free state. But they are so close to each other that their influence cancels each other out. But when such a molecule is introduced into the field, the charges will shift some distance. Thus, a dipole is formed. Such molecules are not affected by thermal motion and, therefore, polarization in them does not depend on temperature.
Figure 2 - polarization of a non-polar dielectric
Charges on the surface of dielectrics, unlike charges induced in conductors, cannot be separated from the surface. When the electric field is removed, the polarization will disappear. The charges will again be redistributed in the volume of the substance. The field strength cannot be increased indefinitely. Since at a certain value the charges will shift so much that a structural change in the material will occur, in other words, a breakdown of the dielectric. In this case, it loses its insulating properties.
Superconductivity
The property of a material to have zero electrical resistance at a temperature below a certain value is called superconductivity.
For some conductive substances, this ability occurs at cold temperatures close to the chemical state of liquid helium.
In 1986, substances with high-temperature superconductivity were discovered. For example, ceramics made of oxygen, barium, copper, lanthanum do not conduct current under normal conditions, but due to heating they become a superconductor.
In practice, substances are used that transmit electric current at 58 degrees Kelvin or more, that is, at a temperature above the boiling point of nitrogen.
Solid high-temperature superconductors are most often used. Liquid and gaseous ones are used less frequently. All these materials are necessary for the manufacture of modern electrical devices of various capacities.