Electric charge
Electrical phenomena have been known since ancient times. Even in ancient Greece (7th century BC), they noticed that if amber was rubbed on wool, it would attract various light objects.
Later, V. Gilbert (XVI century) discovered that, in addition to amber, porcelain and many other bodies, previously rubbed with leather or other soft materials, have the property of attracting light objects. V. Gilbert called this phenomenon electrification
(electron in Greek is amber).
Bodies capable of such interactions are said to be electrically charged
, i.e. they are given an electric charge.
Electric charge q
is a physical scalar quantity that characterizes the ability of bodies to participate in electromagnetic interactions.
Electric charge is denoted by the letters q
or
Q.
_ In the International System of Units (SI), the unit of charge, the coulomb, is established using the unit of current:
- 1 coulomb (C) is the charge passing through the cross-section of a conductor in 1 s at a current of 1 A.
It is impossible to impart a charge of 1 C to a small body, so most often we will use multiples:
1 µC = 1⋅10–6 C, 1 nC = 1⋅10–9 C.
- *Two charges of 1 C each at a distance of 1 km would repel each other with a force slightly less than the force with which the globe attracts a load weighing 1 ton. Therefore, repelling each other, charged particles would not be able to stay on such a body.
A charge of 1 C is very large. But in a conductor that is generally neutral, it is not difficult to set a charge of 1 C in motion. Indeed, in an ordinary light bulb with a power of 100 W at a voltage of 127 V, a current is established that is slightly less than 1 A. At the same time, in 1 s a charge almost equal to 1 C passes through the cross-section of the conductor.
An electrometer is used to detect and measure electrical charges.
. An electrometer consists of a metal rod and a pointer that can rotate around a horizontal axis (Fig. 1). The rod with the arrow is fixed in a plexiglass sleeve and placed in a cylindrical metal case, closed with glass covers.
Rice. 1
Let's touch the positively charged rod to the electrometer rod. We will see that the electrometer needle deviates by a certain angle (see Fig. 1). The rotation of the arrow is explained by the fact that when a charged body comes into contact with the electrometer rod, electrical charges are distributed along the arrow and the rod. Repulsive forces acting between like electric charges on the rod and the pointer cause the pointer to rotate. Let's electrify the ebonite rod again and touch the electrometer rod with it again. Experience shows that with increasing electric charge on the rod, the angle of deviation of the arrow from the vertical position increases. Consequently, by the angle of deflection of the electrometer needle, one can judge the value of the electric charge transferred to the electrometer rod.
Just as in mechanics the concept of a material point is often used, which makes it possible to significantly simplify the solution of many problems, in electrostatics the concept of a “point charge” is used.
- A point charge
is a charged body whose dimensions are significantly less than the distance from this body to the point of observation and other charged bodies.
In particular, if they talk about the interaction of two point charges, they thereby assume that the distance between the two charged bodies under consideration is significantly greater than their linear dimensions.
Properties of electric charge
The totality of all known experimental facts allows us to highlight the following properties of the charge:
- There are two types of electric charges, conventionally called positive and negative. Positively
charged bodies are those that act on other charged bodies in the same way as glass electrified by friction with silk.
Bodies that act in the same way as ebonite electrified by friction with wool are called negatively The choice of the name “positive” for charges arising on glass, and “negative” for charges on ebonite, is completely random.
See also
- Kikoin A.K. Two types of electricity (From the history of physics) // Quantum. - 1984. - No. 1. - P. 34-36
- Charges can be transferred (for example, by direct contact) from one body to another. Unlike body mass, electric charge is not an inseparable characteristic of a given body. The same body under different conditions can have a different charge. But charge does not exist without a body.
- Charges interact with each other: like
charges
repel
,
unlike charges attract
. - Electric charge is discrete
.
This means that there is some smallest, universal, indivisible elementary charge, so that the charge q
of any body is a multiple of this elementary charge: \(~q = N \cdot e\) , where
N
is an integer number of charges,
e
= 1 .6∙10-19 C – the value of the elementary charge.
An example of a particle with an elementary positive charge is a proton
, and an example of a particle with an elementary negative charge is
an electron
.
*Since the value of the elementary charge is very small, for most of the charged bodies observed and used in practice, the number N
is so large that the discrete nature of the charge change does not appear. Therefore, it is believed that under normal conditions the electric charge of bodies changes almost continuously. - In an electrically neutral body, the number of protons and electrons is the same and they are evenly distributed throughout the volume. If the number of electrons in a body is less than the number of protons, then it is positively charged
, and if there is an excess of electrons, then the body
is negatively charged
.
It is this excess charge that is called the charge of the body
:
q
= (
N
p -
N
e)
e
, where
N
p is the number of protons,
N
e is the number of electrons.
- The law of conservation of electric charge
in a closed system, the algebraic sum of electric charges remains constant for any interactions within it: \(~q_1 + q_2 + \ldots + q_n = \operatorname{const}\) .
An isolated (or closed) system
is a system of bodies in which no electrical charges are added or removed from it.
Nowhere and never in nature does an electric charge of the same sign appear or disappear. The appearance of a positive electric charge is always accompanied by the appearance of an equal negative charge. Neither positive nor negative charge can disappear separately; they can only mutually neutralize each other if they are equal in modulus.
The reason for the conservation of electric charge is still unknown.
Electric charge. Interaction of electric charges. Coulomb's law
It is better to start getting acquainted with the phenomena of electrostatics in dry weather. Combing your hair or taking off your sweater, you can observe tiny sparks and faint crackling sounds in the dark. If you rub a plastic comb on your hair and hold it close to small pieces of paper, they will begin to be attracted to the comb.
Electrification is a physical phenomenon that leads to interaction (attraction or repulsion) of two bodies, for example, when they are brought into close contact or by friction (glass and leather, plexiglass and wool, rubber and wool). Discovered in Ancient Greece by rubbing amber (in Greek - “electron”) on wool.
The interaction of electrified bodies at rest is called electrostatic interaction .
Experiments on the interaction of charged bodies have shown that in nature there are two types of charge. B. Franklin called one of them positive, and the other negative. Like charges attract, and like charges repel.
The following types of electrification are distinguished:
- Friction.
- By contact.
- Through influence
- When irradiated.
When bodies are electrified by friction, both bodies involved in the electrification are always simultaneously charged (for example, glass and silk). Moreover, one of them acquires a positive charge, and the other – a negative one. If before electrification both bodies were not charged, then the magnitude of the positive charge of the first body turns out to be exactly equal to the magnitude of the negative charge of the second body.
Modern theory explains the electrification of solids as the movement of electrons that make up the atoms of any body from one body to another.
The nucleus contains positively charged elementary particles - protons. On a body that acquires a negative charge, an excess number of electrons is formed compared to the number of protons, and on a positively charged body there is a deficiency of electrons compared to the number of protons.
Electric charge is a characteristic of a charged body. The minimum charge is designated by the letter e and is equal to 1.6·10 –19 C. An electron and a proton have this charge. The first, most accurate determinations of the electron charge were made by the American scientist R. Millikan and the Russian physicist A. F. Ioffe.
An electrometer is used to detect and measure electrical charge. It is fashionable to judge the amount of charge by the angle of deflection of the arrow.
A decrease in the number of electrons in one body is equal to an increase in their number in another. In this case, the total charge of such a system does not change, remaining equal to zero.
The conservation of the number of protons and electrons on bodies in contact explains the law of charge conservation, confirmed by experience: in an electrically closed system, the algebraic sum of charges does not change.
A quantitative study of the interaction of charged bodies was carried out in 1785 by the French physicist C. Coulomb (1736-1806). He studied the interaction of small charged metal balls using torsion balances.
A glass rod with two metal balls at the ends was suspended from a thin wire. One ball was given an electric charge. A motionless ball charged with the same charge was placed next to it. Based on the angle of rotation of the glass rod, Sh. Coulomb determined the force of interaction. The distance was measured between the centers of the balls.
The modulus of the interaction force F12 between two stationary point electric charges q1 and q2 in a vacuum is proportional to the product of the moduli of these charges and inversely proportional to the square of the distance R12 between them.
A point charge is a model of real charged bodies, the size of which is much smaller than the distance between them.
If there is a system of point charges, then the force acting on each of them is defined as the vector sum of the forces acting on a given charge from all other charges in the system. In this case, the force of interaction of a given charge with a specific charge is calculated as if there were no other charges.
The strength of interaction between point charges depends on the properties of the medium in which they are located:
The properties of the medium are determined by the dielectric constant of the medium ε.
Limits of applicability of Coulomb's law:
- for point charges
- for stationary charges
- valid up to distances of at least 10 -15 m
Application of electrification
1.Electric filters.
To clean the air from dust, for example, in the production of cement, and to clean smoke particles at thermal power plants, electric precipitators are used. Electrified dust particles are attracted to a charged element inside the filter.
2. Uniform spraying of paint with a spray gun.
Electrostatic painting is used to coat metal surfaces, for example in an auto body paint shop. To ensure uniform spraying of paint, a negative charge is applied to the spray gun, and a positive charge is given to the car body. Negatively charged droplets of paint are evenly distributed over the surface of the body, forming a durable, even layer.
3. Making sandpaper.
4. Van de Graaff high voltage generator.
Electrification has found practical application in science and technology. Until recently, the van der Waals generator was widely used in nuclear research at particle accelerators. With its help, it was possible to generate voltages of up to several million volts. The generator was developed in 1929 by American physicist Robert Van der Waals. Friction electrification is used. The charge is transferred on a moving belt and is repeatedly removed from it onto a hollow metal conductor.
5. Grain cleaning.
6. Fingerprinting.
7. Laser printer and copier.
The electrification of bodies during irradiation has found application in photocopying and laser printing.
8. Medicine.
When a Chizhevsky chandelier operates, a large number of negative oxygen ions are formed. When air is inhaled, oxygen ions give off electrical charges to red blood cells and then to cells. As a result, metabolism in the body improves.
Electrification of the body
In order to obtain an electrically charged macroscopic body or, as they say, to electrify
it, you need to separate part of the negative charge from the positive charge associated with it.
- The easiest way to do this is using friction
. If you run a comb through your hair, a small part of the most mobile charged particles - electrons - will move from the hair to the comb and charge it negatively, and the hair will become positively charged. When electrified by friction, both bodies acquire charges of opposite sign, but equal in magnitude.
- Another way to electrify bodies is by exposing them to various radiations
(in particular, ultraviolet, x-rays and
γ
-rays). This method is most effective for electrifying metals, when, under the influence of radiation, electrons are knocked out from the surface of the metal and the conductor acquires a positive charge.
- Electrification through influence or " electrical induction
". When a positive charge is applied to a conductor, electrons are attracted to it and accumulate at the nearest end of the conductor. A certain number of “excess” electrons appear on it, and this part of the conductor becomes negatively charged. At the far end there is a lack of electrons and, therefore, an excess of positive ions: a positive charge appears here. When a negatively charged body is brought close to a conductor, electrons accumulate at the far end, and an excess of positive ions is produced at the near end. After removing the charge that causes the movement of electrons, they are again distributed throughout the conductor, so that all parts of it are still uncharged. Induced charges can be separated if, in the presence of a charged body, the conductor is divided into parts. In this case, the displaced electrons can no longer return back after the removal of the external charge.
*Friction electrification mechanism
It is very simple to electrify bodies using friction. But explaining how this happens turned out to be a very difficult task.
1 version
. When electrifying bodies, close contact between them is important. Electrical forces hold electrons inside the body. But for different substances these forces are different. During close contact, a small part of the electrons of the substance in which the connection of electrons with the body is relatively weak passes to another body. The electron movements do not exceed the interatomic distances (10-8 cm). But if the bodies are separated, then both of them will be charged. Since the surfaces of bodies are never perfectly smooth, the close contact between bodies necessary for transition is established only on small areas of the surfaces. When bodies rub against each other, the number of areas with close contact increases, and thereby the total number of charged particles passing from one body to another increases. But it is not clear how electrons can move in such non-conducting substances (insulators) as ebonite, plexiglass and others. They are bound in neutral molecules.
2 version
. Using the example of an ionic LiF crystal (insulator), this explanation looks like this. During the formation of a crystal, various types of defects arise, in particular vacancies - unfilled spaces at the nodes of the crystal lattice. If the number of vacancies for positive lithium ions and negative fluorine ions is not the same, then the crystal will be charged in volume upon formation. But the charge as a whole cannot be retained by the crystal for long. There is always a certain amount of ions in the air, and the crystal will pull them out of the air until the charge of the crystal is neutralized by a layer of ions on its surface. Different insulators have different space charges, and therefore the charges of the surface layers of ions are different. During friction, the surface layers of ions are mixed, and when the insulators are separated, each of them becomes charged.
Can two identical insulators
, for example the same LiF crystals? If they have the same own space charges, then no. But they can also have different own charges if the crystallization conditions were different and a different number of vacancies appeared. As experience has shown, electrification during friction of identical crystals of ruby, amber, etc. can actually occur. However, the above explanation is unlikely to be correct in all cases. If bodies consist, for example, of molecular crystals, then the appearance of vacancies in them should not lead to charging of the body.
Electrification
To understand how a body acquires and retains an electrical charge, we first need to take a closer look at the proton and electron. The proton is lazy and clumsy - it certainly won’t move anywhere unless we move the entire atom.
But the electron is a mobile guy, and it costs nothing for him to move from one atom to another.
We will talk about two types of electrification: electrification by contact and electrification by friction.
- Electrification by contact is a process in which we take two conducting bodies: negatively charged and neutral.
Free electrons move from an uncharged body to a neutral one. And if we take a positively charged body instead of a negative one, then free electrons will move from the neutral body to balance the charges.
- Electrification by friction is when we take two uncharged bodies and rub them against each other.
Electrons move from one body to another and, unlike electrification, by contact they are charged with charges of opposite sign and equal in magnitude.
That is, upon contact, the charge is distributed of the same sign and equally. It’s like sharing candy with a friend that you have in abundance.
With friction, on the contrary, the charges on the bodies will be of different signs, but also in the same quantity. For example, you have an equal amount of money in rubles and dollars, and I have a similar situation with the same amount. You decided to fly to the USA, but I don’t need dollars. In order not to go to the bank, we can simply exchange. Then you will only have dollars, and I will only have rubles. The main thing is to agree on the course 
Let's solve a couple of problems on this topic.
Problem one
What material can be made of the rod connecting the electrometers shown in the figure?
A. Glass
B. Ebonite
Solution:
It can be made of either a conductor or a dielectric. A conductor allows charges to pass through itself, but a dielectric does not. If we look at the readings of the electrometers, we will see that they are different.
As we remember, upon contact, the charges are equal in magnitude (one electrometer shares candies with another). In this case, no one shared it with anyone, which means that the rod does not let through - it is a dielectric. Both glass and hard rubber are dielectrics. So both options are suitable!
Problem two
In the process of rubbing against the silk, the glass ruler acquired a positive charge. How did the number of charged particles on the ruler and silk change, provided that no exchange occurred during friction?
A) the number of protons on a glass ruler
B) the number of electrons on silk
Solution:
Remember how we characterized the proton: it is lazy and motionless! This means that the number of protons either on a glass ruler or on silk simply cannot change. We do not break off a piece of the ruler along with the atoms of which it consists. But electrons willingly move. We know that the ruler has acquired a positive charge. It turns out that the electrons escaped from her to the silk. Consequently, the number of electrons on the silk increased.
Coulomb's law
In 1785, the French physicist Charles Coulomb experimentally established the basic law of electrostatics - the law of interaction of two stationary point charged bodies or particles.
The law of interaction of stationary electric charges - Coulomb's law - is a basic (fundamental) physical law and can only be established experimentally. It does not follow from any other laws of nature.
If we denote the charge modules by | q
1|
and | q
2|, then Coulomb’s law can be written in the following form:
\(~F = k \cdot \dfrac{|q_1| \cdot |q_2|}{r^2}\) , (1)
where k
– proportionality coefficient, the value of which depends on the choice of units of electric charge. In the SI system \(~k = \dfrac{1}{4 \pi \cdot \varepsilon_0} = 9 \cdot 10^9\) N m2/C2, where ε0 is the electrical constant equal to 8.85 10- 12 C2/N m2.
- the force of interaction between two point stationary charged bodies in a vacuum is directly proportional to the product of the charge modules and inversely proportional to the square of the distance between them.
This force is called Coulomb force
.
Coulomb's law in this formulation is valid only for point
charged bodies, because only for them the concept of distance between charges has a certain meaning. There are no point charged bodies in nature. But if the distance between the bodies is many times greater than their size, then neither the shape nor the size of the charged bodies significantly, as experience shows, affects the interaction between them. In this case, the bodies can be considered as point bodies.
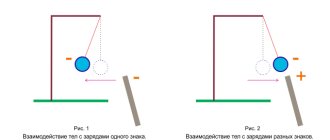
It is easy to find that two charged balls suspended on threads either attract each other or repel each other. It follows that the forces of interaction between two stationary point charged bodies are directed along the straight line connecting these bodies. Such forces are called central
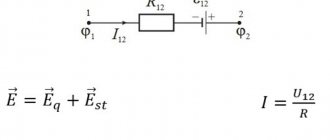
. If we denote by \(~\vec F_{12}\) the force acting on the first charge from the second, and by \(~\vec F_{21}\) the force acting on the second charge from the first (Fig. 2 , 3), then, according to Newton’s third law, \(~\vec F_{12} = -\vec F_{21}\) .
- Rice. 2
- Rice. 3
Knowing the law of interaction of point charged bodies, one can calculate the force of interaction of any charged bodies. To do this, bodies must be mentally broken down into such small elements that each of them can be considered a point. By adding geometrically the forces of interaction of all these elements with each other, we can calculate the resulting interaction force.
The discovery of Coulomb's law is the first concrete step in studying the properties of electric charge. The presence of an electric charge in bodies or elementary particles means that they interact with each other according to Coulomb's law. No deviations from the strict implementation of Coulomb's law have currently been detected.
Coulomb's law is also valid for charged balls at any distance between their centers, if the volume or surface charge density of each of them is constant. (Note that, unlike gravitational interaction, electrostatic interaction can lead to attraction and repulsion of bodies.)
see also
- Coulomb's law
Literature
- Aksenovich L. A. Physics in secondary school: Theory. Tasks. Tests: Textbook. allowance for institutions providing general education. environment, education / L. A. Aksenovich, N. N. Rakina, K. S. Farino; Ed. K. S. Farino. - Mn.: Adukatsiya i vyakhavanne, 2004. - P. 209-210, 211-214.
- Zhilko, V.V. Physics: textbook. allowance for 11th grade. general education institutions with Russian language training with a 12-year period of study (basic and advanced levels) /V. V. Zhilko, L. G. Markovich. — 2nd ed., revised. — Minsk: Nar. Asveta, 2008. - pp. 72-79.